Some examples of interactions between certain rare earth elements and soil Scientific paper
Main Article Content
Abstract
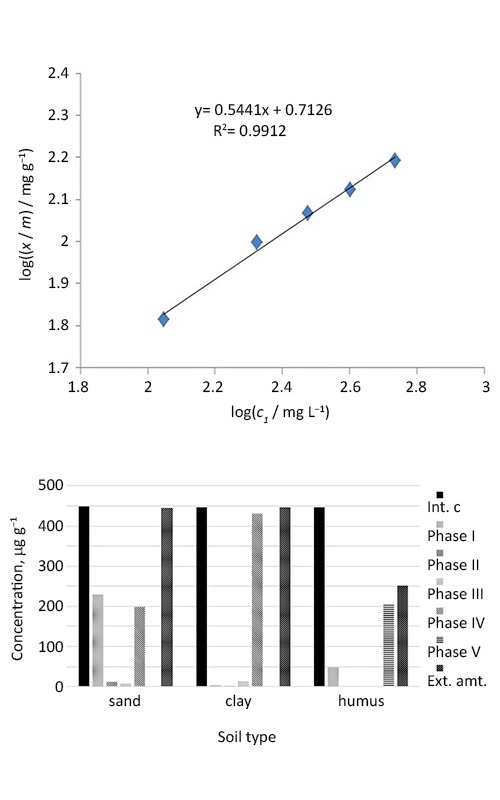
The rare earth elements represent an increasingly more and more important industrial resource. The increased use may result in waste generation, and their impact upon the environment quality has not been studied sufficiently. Their interaction with soil has been studied in this paper. The Freundlich adsorption isotherm has been determined for lanthanum, erbium and gadolinium at three different soil types (humus, clay and sand type), whereas the sequential extraction at these soil types has been applied for lanthanum and neodymium. The interaction of certain rare earth elements with soil components has been tested as well as the quantity in which these elements are bound to soil and later on extracted in solutions. The objective was to determine the soil capacity for disposal, first of all, of the electronic waste that contains these elements and to assume their fate in the environment.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
I. Anastopoulos, A. Bhatnagar, E. C. Lima, J. Mol. Liq. 221 (2016) 954 (https://doi.org/10.1016/j.molliq.2016.06.076)
F. Tommasi, P. J. Thomas, G. Pagano, G. A. Perono, R. Oral, D. M. Lyons, M. Toscanesi, M. Trifuoggi, J. Arch. Environ. Contam. Toxicol. 81 (2021) 531 (https://doi.org/10.1007/s00244-020-00773-4)
V. Balaram, J. Geosci. Front. 10 (2019) 1285
V. M. Goldschmidt, J. Chem. Soc. (Resumed) (1937) 655 (https://doi.org/10.1039/JR9370000655)
M. Bernat, Cah. ORSTOM Ser. Geol 7 (1975) 65 (https://core.ac.uk/download/pdf/39881849.pdf)
V. Zepf, in: Rare Earth Elements, Springer, Berlin, 2013, pp. 11–39 (https://doi.org/10.1007/978-3-642-35458-8_2)
Y. Takaya, K. Yasukawa, T. Kawasaki, K. Fujinaga, J. Ohta, Y. Usui, K. Nakamura, J. Kimura, Q. Chang, M. Hamada, G. Dodbiba, T. Nozaki, K. Iijima, T. Morisawa, T. Kuwahara, Y. Ishida, T. Ichimura, M. Kitazume, T. Fujita, Y. Kato, Sci. Rep. 8 (2018) (https://doi.org/10.1038/s41598-018-23948-5)
Y. Kato, K. Fujinaga, K. Nakamura, Y. Takaya, K. Kitamura, J. Ohta, R. Toda, T. Nakashima, H. Iwamori, Nature Geosci. 4 (2011) 535 (https://doi.org/10.1038/ngeo1185)
Y. Wan, C. Liu, J. Rare Earths 23 (2005) 377
G. W. Beall, B. H. Ketelle, R. G. Haire, G. D. O'Kelley, ACS Symp. Ser. 100 (1979) 201 (http://dx.doi.org/10.1021/bk-1979-0100.ch012)
F. Coppin, G. Berger, A. Bauer, S. Castet, M. Loubet, Chem. Geology 182 (2002) 57 (https://doi.org/10.1016/S0009-2541(01)00283-2)
IAEA, Radiation protection and NORM residue management in the production of rare earths from thorium containing minerals, Safety Reports Series 68 (2011) 259
G. Haxel, US Geological Survey 87 (2002) (https://pubs.usgs.gov/fs/2002/fs087-02)
C. Ng, J. N. Losso, W. E. Marshall, R. M. Rao, Biores. Technol. 85 (2002) 131 (https://doi.org/10.1016/S0960-8524(02)00093-7)
S. McLeod, Notes Soil Tech. (1973) 73
D. P. Gangwar, M. Baskar, Texture determination of soil by hydrometer method for forensic purpose, Central Forensic Science Laboratory, Chandigarh, 2019 (http://dx.doi.org/10.13140/RG.2.2.16057.60001)
J. Isailović, Master Thesis, Faculty of Chemistry, Belgrade, 202) (in Serbian)
Z. Nikolovski, Master Thesis, Faculty of Chemistry, Belgrade, 2021 (in Serbian)
A. Guša, M. Đolić, B. Lekić, V. Rajaković-Ognjanović, Water Manage. 47 (2015) 67 (http://grafar.grf.bg.ac.rs/handle/123456789/656) (in Serbian)
EPA M 3051A, Microwave assisted acid digestion of sediments, sludges, soils and oils, 2007
B. Stanimirović, J. Otašević, J. Petrović, D. Savić, D. Tonić, A. Šestak, Lj. Miličić, M. Savić, S. Jovanović, Possibilities of extraction of high-value useful ions and ionic species from ash TPP JP EPS, Institute MOL d.o.o. Stara Pazova, Institute IMS a.d., Belgrade, 2016, p. 33 (in Serbian)
D. L. Jensen, A. Ledin, T. H. Christensen, Water Res. 33 (1999) 2642 (https://doi.org/10.1177%2F0734242X04042146)
J. U. Keller, R. Staudt, Gas adsorption equilibria: experimental methods and adsorptive isotherms, Springer Science & Business Media, 2005, pp. 1–402 (ISBN 978-0-387-23598-1)
A. S. Erses, M. A. Fazal, T. T. Onay, W. H. Craig, J. Hazard. Mater. 121 (2005 ) 223 (https://doi.org/10.1016/j.jhazmat.2005.02.011)
S. Veli, B. Alyüz, J. Hazard. Mater. 149 (2007) 226 (https://doi.org/10.1016/j.jhazmat.2007.04.109)
A. Özer, H. B. Pirincci, J. Hazard. Mater. 137 (2006) 849 (https://doi.org/10.1016/j.jhazmat.2006.03.009).