Synthesis, spectroscopic characterization and DFT analysis of dichlorido(η6-p-cymene)ruthenium(II) complexes with isonicotinate-polyethylene glycol ester ligands Scientific paper
Main Article Content
Abstract
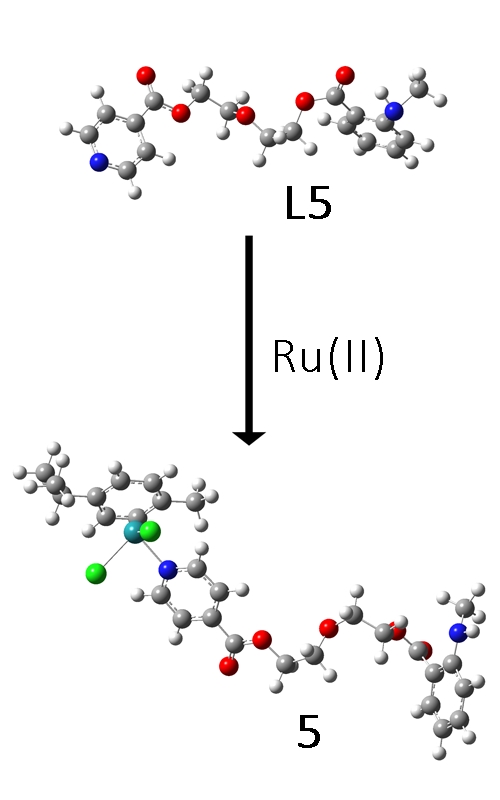
Ruthenium complexes have gained significant attention due to the ruthenium similarity to iron, lower toxicity, and higher anticancer effectiveness than other compounds. In this contribution, five new isonicotinate-polyethylene glycol ester ligands were synthesised and characterised by NMR and IR spectroscopies. The corresponding Ru(II) complexes were also obtained, and their structure was investigated by traditional methods. The optimisation of structures was performed at B3LYP/6-31+G(d,p) level of theory for H, C, N and O atoms and B3LYP/LanL2DZ for Ru. The intramolecular stabilisation interactions were assessed through the natural bond orbital approach. The NMR chemical shifts were predicted by the gauge independent atomic orbital method and compared to the exprimental values. High correlation coefficients and low mean absolute errors between these data sets proved that the predicted structures described well the experimental ones. The theoretical and experimental IR spectra were also compared, and differences in the most notable bands were described. One of the ligands (L5) and complexes (5) showed fluorescent properties due to methylisatoic moiety. The electronic spectra of this compound were modelled by the time dependent-density functional theory method. The difference of 11 nm between the experimental and the theoretical wavelength was explained by the interactions between the solvent and the solute. Further biological and theoretical studies are advised for this series of compounds.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
R. G. Kenny, C. J. Marmion, Chem. Rev. 119 (2019) 1058 (https://doi.org/10.1021/acs.chemrev.8b00271)
T. C. Johnstone, K. Suntharalingam, S. J. Lippard, Chem. Rev. 116 (2016) 3436 (https://doi.org/10.1021/acs.chemrev.5b00597)
S. Thota, D. A. Rodrigues, D. C. Crans, E. J. Barreiro, J. Med. Chem. 61 (2018) 5805 (https://doi.org/10.1021/acs.jmedchem.7b01689)
A. Rilak Simović, R. Masnikosa, I. Bratsos, E. Alessio, Coord. Chem. Rev. 398 (2019) 113011 (https://doi.org/10.1016/j.ccr.2019.07.008)
L. Conti, E. Macedi, C. Giorgi, B. Valtancoli, V. Fusi, Coord. Chem. Rev. 469 (2022) 214656 (https://doi.org/10.1016/j.ccr.2022.214656)
S. Y. Lee, C. Y. Kim, T.-G. Nam, Drug Des. Devel. Ther. 14 (2020) 5375 (https://doi.org/10.2147/DDDT.S275007)
B. Therrien, Coord. Chem. Rev. 253 (2009) 493 (https://doi.org/10.1016/j.ccr.2008.04.014)
M. J. Clarke, Coord. Chem. Rev. 232 (2002) 69 (https://doi.org/10.1016/S0010-8545(02)00025-5)
G. S. Smith, B. Therrien, Dalt. Trans. 40 (2011) 10793 (https://doi.org/10.1039/C1DT11007A)
D. S. Dimić, G. N. Kaluđerović, E. H. Avdović, D. A. Milenković, M. N. Živanović, I. Potočňák, E. Samoľová, M. S. Dimitrijević, L. Saso, Z. S. Marković, J. M. Dimitrić Marković, Int. J. Mol. Sci. 23 (2022) 1001 (https://doi.org/10.3390/ijms23021001)
G. Jia, G.-L. Law, K.-L. Wong, P. A. Tanner, W.-T. Wong, Inorg. Chem. 47 (2008) 9431 (https://doi.org/10.1021/ic8010103)
R. Schobert, B. Biersack, Inorg. Chim. Acta 358 (2005) 3369 (https://doi.org/10.1016/j.ica.2005.05.015)
L. D. Ramos, G. Cerchiaro, K. P. Morelli Frin, Inorg. Chim. Acta 501 (2020) 119329 (https://doi.org/10.1016/j.ica.2019.119329)
C.-M. Wang, Y.-L. Chuang, S.-T. Chuang, K.-H. Lii, J. Solid State Chem. 177 (2004) 2305 (https://doi.org/10.1016/j.jssc.2004.02.030)
J.-Y. Kim, A. J. Norquist, D. O’Hare, Chem. Mater. 15 (2003) 1970 (https://doi.org/10.1021/cm021722n)
T. Eichhorn, E. Hey-Hawkins, D. Maksimović-Ivanić, M. Mojić, J. Schmidt, S. Mijatović, H. Schmidt, G. N. Kaluderović, Appl. Organomet. Chem. 29 (2015) 20 (https://doi.org/10.1002/aoc.3238)
Gaussian 09, Revision A.02, Gaussian Inc., Wallingford, CT, 2009
A. D. Becke, J. Chem. Phys. 98 (1993) 5648 (https://doi.org/10.1063/1.464913)
T. H. Dunning, J. Chem. Phys. 90 (1989) 1007 (https://doi.org/10.1063/1.456153)
P. J. Hay, W. R. Wadt, J. Chem. Phys. 82 (1985) 299 (https://doi.org/10.1063/1.448975)
P. J. Hay, W. R. Wadt, J. Chem. Phys. 82 (1985) 270 (https://doi.org/10.1063/1.448799)
R. Dennington, K. Todd, J. Millam, Gauss View, Version 5, Semichem Inc., Shawnee, KS, 2009
A. V. Marenich, C. J. Cramer, D. G. Truhlar, J. Phys. Chem., B 113 (2009) 6378 (https://doi.org/10.1021/jp810292n)
A. E. Reed, R. B. Weinstock, F. Weinhold, J. Chem. Phys. 83 (1985) 735 (https://doi.org/10.1063/1.449486)
R. Zieliński, H. Szymusiak, Pol. J. Food Nutr. Sci. 12 (2003) 157 (http://journal.pan.olsztyn.pl/pdf-98612-30420?filename=APPLICATION%20OF%20DFT.pdf)
J. A. Bohmann, F. Weinhold, T. C. Farrar, J. Chem. Phys. 107 (1997) 1173 (https://doi.org/10.1063/1.474464)
D. Jacquemin, J. Preat, V. Wathelet, E. A. Perpète, Chem. Phys. 328 (2006) 324 (https://doi.org/10.1016/j.chemphys.2006.07.037)
G. R. Newkome, K. J. Theriot, V. K. Gupta, R. N. Balz, F. R. Fronczek, Inorg. Chim. Acta 114 (1986) 21 (https://doi.org/10.1016/S0020-1693(00)84582-X)
C. O. Badgett, C. F. Woodward, J. Am. Chem. Soc. 69 (1947) 2907 (https://doi.org/10.1021/ja01203a501)
H. H. Bosshard, R. Mory, M. Schmid, H. Zollinger, Helv. Chim. Acta 42 (1959) 1653 (https://doi.org/10.1002/hlca.19590420526)
J. Clayden, N. Greeves, S. Warren, Organic Chemistry, 2nd ed., Oxford University Press, Oxford, 2012 (ISBN: 978-0-19-927029-3)
A. Wollrab, Organische Chemie, 2nd ed., Springer Verlag, Berlin, 2012 (ISBN:978-3-642-45143-0)
Organikum, 22nd ed., Wiley VCH, Weinheim, 2004 (ISBN: 978-3-527-33968-6)
G. Świderski, M. Kalinowska, R. Świsłocka, S. Wojtulewski, W. Lewandowski, Spectrochim. Acta, A 100 (2013) 41 (https://doi.org/10.1016/j.saa.2012.02.047)
S. Ramalingam, S. Periandy, S. Mohan, Spectrochim. Acta, A 77 (2010) 73 (https://doi.org/10.1016/j.saa.2010.04.027))
A. G. Medvedev, A. A. Mikhailov, P. V. Prikhodchenko, T. A. Tripol’skaya, O. Lev, A. V. Churakov, Russ. Chem. Bull. 62 (2013) 1871 (https://link.springer.com/article/10.1007/s11172-013-0269-9)
M. Al-Noaimi, M. A. AlDamen, Inorg. Chim. Acta 387 (2012) 45 (https://doi.org/10.1016/j.ica.2011.12.050)
V. Uahengo, P. Cai, J. Naimhwaka, A. Rahman, L. S. Daniel, H. Bhakhoa, L. Rhyman, P. Ramasami, Polyhedron 173 (2019) 114106 (https://doi.org/10.1016/j.poly.2019.114106)
A. A. Sikalov, V. V. Pavlovskiy, A. A. Kirilchuk, Inorg. Chem. Commun. 99 (2019) 156 (https://doi.org/10.1016/j.inoche.2018.11.020)
T. Eichhorn, F. Kolbe, S. Mišić, D. Dimić, I. Morgan, M. Saoud, D. Milenković, Z. Marković, T. Rüffer, J. Dimitrić Marković, G. N. Kaluđerović, Int. J. Mol. Sci. 24 (2023) 689 (https://doi.org/10.3390/ijms24010689)
A. Mondal, U. Sen, N. Roy, V. Muthukumar, S. K. Sahoo, B. Bose, P. Paira, Dalt. Trans. 50 (2021) 979 (https://doi.org/10.1039/D0DT03107K)
M. T. Rupp, N. Shevchenko, G. S. Hanan, D. G. Kurth, Coord. Chem. Rev. 446 (2021) 214127 (https://doi.org/10.1016/j.ccr.2021.214127)
E. Pretsch, P. Bühlmann, C. Affolter, Structure Determination of organic compounds, 3rd ed., Springer Verlag, Berlin, 2000 (ISBN: 978-3-662-62439-5)
M. Hesse, H. Meier, B. Zeeh, Spektroskopische Methoden in der organischen Chemie, 7th ed., Georg Thieme Verlag, Stuttgart, 2002 (http://dx.doi.org/10.1055/b-002-46985)
J. R. Durig, W. A. McAllister, E. E. Mercer, J. Inorg. Nucl. Chem. 29 (1967) 1441 (https://doi.org/10.1016/0022-1902(67)80244-6)
I. Chevrier, J. L. Sagué, P. S. Brunetto, N. Khanna, Z. Rajacic, K. M. Fromm, Dalton Trans. 42 (2013) 217 (https://doi.org/10.1039/C2DT31259J)
D. Roell, T. W. Rösler, S. Degen, R. Matusch, A. Baniahmad, Chem. Biol. Drug Des. 77 (2011) 450. (https://doi.org/10.1111/j.1747-0285.2011.01116.x).