Evaluation of antitumor potential of Cu(II) complex with hydrazone of 2-acetylthiazole and Girard’s T reagent Scientific paper
Main Article Content
Abstract
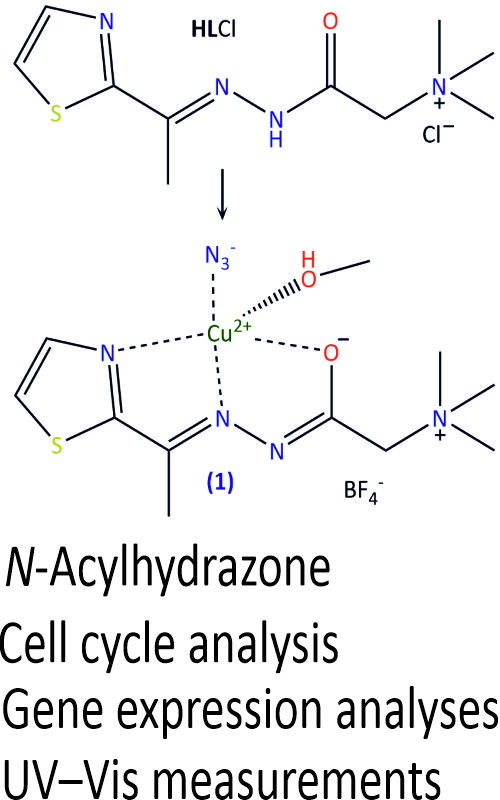
In this paper, the previously synthesized Cu(II) complex ([CuL1(N3) (CH3OH)]BF4) with N,N,N-trimethyl-2-oxo-2-(2-(1-(thiazol-2-yl)ethylidene)hydrazinyl)ethan-1-aminium chloride, has been characterized and its biological activity has been studied in detail. The Cu(II) complex consists of ligand coordinated in a deprotonated, formally neutral zwitter-ionic form, via NNO atoms, one azido ligand and one methanol molecule. The Cu(II) complex was selected due to results of the cytotoxic activity, the brine shrimp test and DPPH radical scavenging activity, which were previously performed. The effects of Cu(II) complex on cell cycle phase distribution of cervical adenocarcinoma HeLa cells were investigated in order to examine the mechanisms of its anticancer activity. The measurement of intracellular ROS levels in HeLa and HaCaT cell lines were evaluated in order to explore their possible generation and the role in cytotoxic activity. The possible anti-invasive and anti-angiogenic properties of Cu(II) complex were evaluated. DNA binding experiments, including fluorescence displacement study and DNA cleavage experiments, were performed in order to obtain information on the type of DNA–metal complex interactions.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
M. Çınarlı, Ç. Yüksektepe Ataol, E. Çınarlı, Ö. İdil, J. Mol. Struc. 1213 (2020) 128 (http://dx.doi.org/10.1016/j.molstruc.2020.128152)
F. I. Abouzayed, S. M. Emam, S. A. Abouel-Enein, J. Mol. Struc. 1216 (2020) 128 (http://dx.doi.org/10.1016/j.molstruc.2020.128314)
K. Rishu, K. Harpreet, K. Brij Kishore, Sci. Rev. Chem. Comm. 3 (2013) 1 (https://www.tsijournals.com/articles/applications-of-copper--schiffs-base-complexes--a-revie.pdf)
S. Jiang, H. Ni, F. Liu, S. Gu, P. Yu, Y. Gou, Inorg. Chim. Acta 499 (2020) 119186 (http://dx.doi.org/10.1016/j.ica.2019.119186)
S. Yousef Ebrahimipour, I. Sheikhshoaie, A. Crochet, M. Khaleghi, K. M. Fromm, J. Mol. Struc. 1072 (2014) (http://dx.doi.org/10.1016/j.molstruc.2014.05.024)
M. Sutradhar, Rajeshwari, T. Roy Barman, A. R. Fernandes, F. Paradinha, C. Roma-
-Rodrigues, M. F. C. Guedes da Silva, A. J. L. Pombeiro, J. Inorg. Biochem. 175 (2017) (http://dx.doi.org/10.1016/j.jinorgbio.2017.07.034)
Q. Mo, J. Deng, Y. Liu, G. Huang, Z. Li, P. Yu, Y. Gou, F. Yang, Eur. J. Med. Chem. 156 (2018) (http://dx.doi.org/10.1016/j.ejmech.2018.07.022)
S. Y. Ebrahimipour, I. Sheikhshoaie, M. Mohamadi, S. Suarez, R. Baggio, M. Khaleghi, M. Torkzadeh-Mahani, A. Mostafavi, Spectrochim. Acta, A 142 (2015) 410 (http://dx.doi.org/10.1016/j.saa.2015.01.088)
M. M. Fousiamol, M. Sithambaresan, K. K. Damodaran, M. R. P. Kurup, Inorg. Chim. Acta 501 (2020) 119301 (http://dx.doi.org/10.1016/j.ica.2019.119301)
P. H. O. Santiago, M. B. Santiago, C. H. G. Martins, C. C. Gatto, Inorg. Chim. Acta 508 (2020) 119632 (http://dx.doi.org/10.1016/j.ica.2020.119632)
P. H. O. Santiago, F. S. Tiago, M. S. Castro, P. E. N. Souza, J. B. L. Martins, C. C. Gatto, J. Inorg. Biochem. 204 (2020) 110949 (http://dx.doi.org/10.1016/j.jinorgbio.2019.110949)
Y. Gou, J. Li, B. Fan, B. Xu, M. Zhou, F. Yang, Eur. J. Med. Chem. 134 (2017) (http://dx.doi.org/10.1016/j.ejmech.2017.04.026)
D. K. Sau, R. J. Butcher, S. Chaudhuri, N. Saha, Mol. Cell. Biochem. 253 (2003) 21 (http://dx.doi.org/10.1023/A:1026041032078)
O. Palamarciuc, M. N. M. Milunović, A. Sîrbu, E. Stratulat, A. Pui, N. Gligorijevic, S. Radulovic, J. Kožíšek, D. Darvasiová, P. Rapta, E. A. Enyedy, G. Novitchi, S. Shova, V. B. Arion, New. J. Chem. 43 (2019) 134 (http://dx.doi.org/10.1039/C8NJ04041A)
M. R. Milenković, A. T. Papastavrou, D. Radanović, A. Pevec, Z. Jagličić, M. Zlatar, M. Gruden, G. C. Vougioukalakis, I. Turel, K. Anđelković, B. Čobeljić, Polyhedron 165 (2019) 22 (http://dx.doi.org/10.1016/j.poly.2019.03.001)
T. Keškić, B. Čobeljić, M. Gruden, K. Anđelković, A. Pevec, I. Turel, D. Radanović, M. Zlatar, Cryst. Growth Des. 19 (2019) 4810 (http://dx.doi.org/10.1021/acs.cgd.9b00760)
N. Stevanović, P. P. Mazzeo, A. Bacchi, I. Z. Matić, M. Đorđić Crnogorac, T. Stanoj-ković, M. Vujčić, I. Novaković, D. Radanović, M. Šumar-Ristović, D. Sladić, B. Čobeljić, K. Anđelković, J. Biol. Inorg. Chem. (2021) (http://dx.doi.org/10.1007/s00775-021-01893-5)
T. T. Adejumo, N. v. Tzouras, L. P. Zorba, D. Radanović, A. Pevec, S. Grubišić, D. Mitić, K. K. Anđelković, G. C. Vougioukalakis, B. Čobeljić, I. Turel, Molecules 25 (2020) 4043 (http://dx.doi.org/10.3390/molecules25184043)
N. Stevanović, M. Zlatar, I. Novakovic, A. Pevec, D. Radanović, I. Matić, M. Djordjic Crnogorac, T. Stanojkovic, M. Vujčić, M. Gruden, D. Sladić, K. Anđelković, I. Turel, B. Čobeljić, Dalton Trans. (2021) (http://dx.doi.org/10.1039/D1DT03169D)
Michael G. Ormerod, Flow cytometry. A practical approach. 3rd ed., Oxford University Press, Oxford, 2000
E. Aranda, G. I. Owen, Biol. Res. 42 (2009) 377 (http://dx.doi.org/10.4067/S0716-97602009000300012)
I. Z. Matić, I. Aljančić, V. Vajs, M. Jadranin, N. Gligorijević, S. Milosavljević, Z. D. Juranić, Nat. Prod. Commun. 8 (2013) 1291 (https://doi.org/10.1177%2F1934578X1300800927)
M. Č. Romanović, B. Čobeljić, A. Pevec, I. Turel, S. Grubišić, D. Radanović, K. Anđelković, M. Milenković, M. R. Milenković, J. Coord. Chem. 70 (2017) 3702 (http://dx.doi.org/10.1080/00958972.2017.1405262)
R. Vijayalakshmi, M. Kanthimathi, V. Subramanian, B. U. Nair, Biochem. Biophys. Res. Commun. 271 (2000) 731 (http://dx.doi.org/10.1006/bbrc.2000.2707)
P. Nagababu, A. K. Barui, B. Thulasiram, C. S. Devi, S. Satyanarayana, C. R. Patra, B. Sreedhar, J. Med. Chem. 58 (2015) 5226 (http://dx.doi.org/10.1021/acs.jmedchem.5b00651)
M. v. Rodić, V. M. Leovac, L. S. Jovanović, V. Spasojević, M. D. Joksović, T. Stanoj¬ković, I. Z. Matić, L. S. Vojinović-Ješić, V. Marković, Eur. J. Med. Chem. 115 (2016) 75 (http://dx.doi.org/10.1016/j.ejmech.2016.03.003)
R. I. Teleanu, C. Chircov, A. M. Grumezescu, D. M. Teleanu, J. Clin. Med. 9 (2019) 84 (http://dx.doi.org/10.3390/jcm9010084)
L. Strekowski, B. Wilson, Mutat. Res.-Fund. Mol. M . 623 (2007) 3 (http://dx.doi.org/10.1016/j.mrfmmm.2007.03.008)
F. R. Keene, J. A. Smith, J. G. Collins, Coord. Chem. Rev. 253 (2009) 2021 (http://dx.doi.org/10.1016/j.ccr.2009.01.004)
S. E. Sherman, Dan. Gibson, A. H. J. Wang, S. J. Lippard, J. Am. Chem. Soc. 110 (1988) 7368 (http://dx.doi.org/10.1021/ja00230a017)
I. Turel, J. Kljun, Curr. Top. Med. Chem. 11 (2011) 2661 (http://dx.doi.org/10.2174/156802611798040787)
M. Cory, D. D. McKee, J. Kagan, Henry D. W., J. A. Miller, J. Am. Chem. Soc. 107 (1985) 2528 (http://dx.doi.org/10.1021/ja00294a054)
J. Wang, L. Shuai, X. Xiao, Y. Zeng, Z. Li, T. Matsumura-Inoue, J. Inorg. Biochem. 99 (2005) 883 (http://dx.doi.org/10.1016/j.jinorgbio.2004.12.018)
F. Dimiza, S. Fountoulaki, A. N. Papadopoulos, C. A. Kontogiorgis, V. Tangoulis, C. P. Raptopoulou, V. Psycharis, A. Terzis, D. P. Kessissoglou, G. Psomas, Dalton Trans. 40 (2011) 8555 (http://dx.doi.org/10.1039/c1dt10714c)
E. S. Koumousi, M. Zampakou, C. P. Raptopoulou, V. Psycharis, C. M. Beavers, S. J. Teat, G. Psomas, T. C. Stamatatos, Inorg. Chem. 51 (2012) 7699 (http://dx.doi.org/10.1021/ic300739x)
S. Mardanya, S. Karmakar, D. Maity, S. Baitalik, Inorg. Chem. 54 (2015) 513 (http://dx.doi.org/10.1021/ic502271k)
R. Kakkar, R. Garg, Suruchi, J. Mol. Struc.: THEOCHEM 584 (2002) 37 (http://dx.doi.org/10.1016/S0166-1280(02)00026-X).