Reactions of 2-acetylpyridine-aminoguanidine with Cu(II) under different reaction conditions Scientific paper
Main Article Content
Abstract
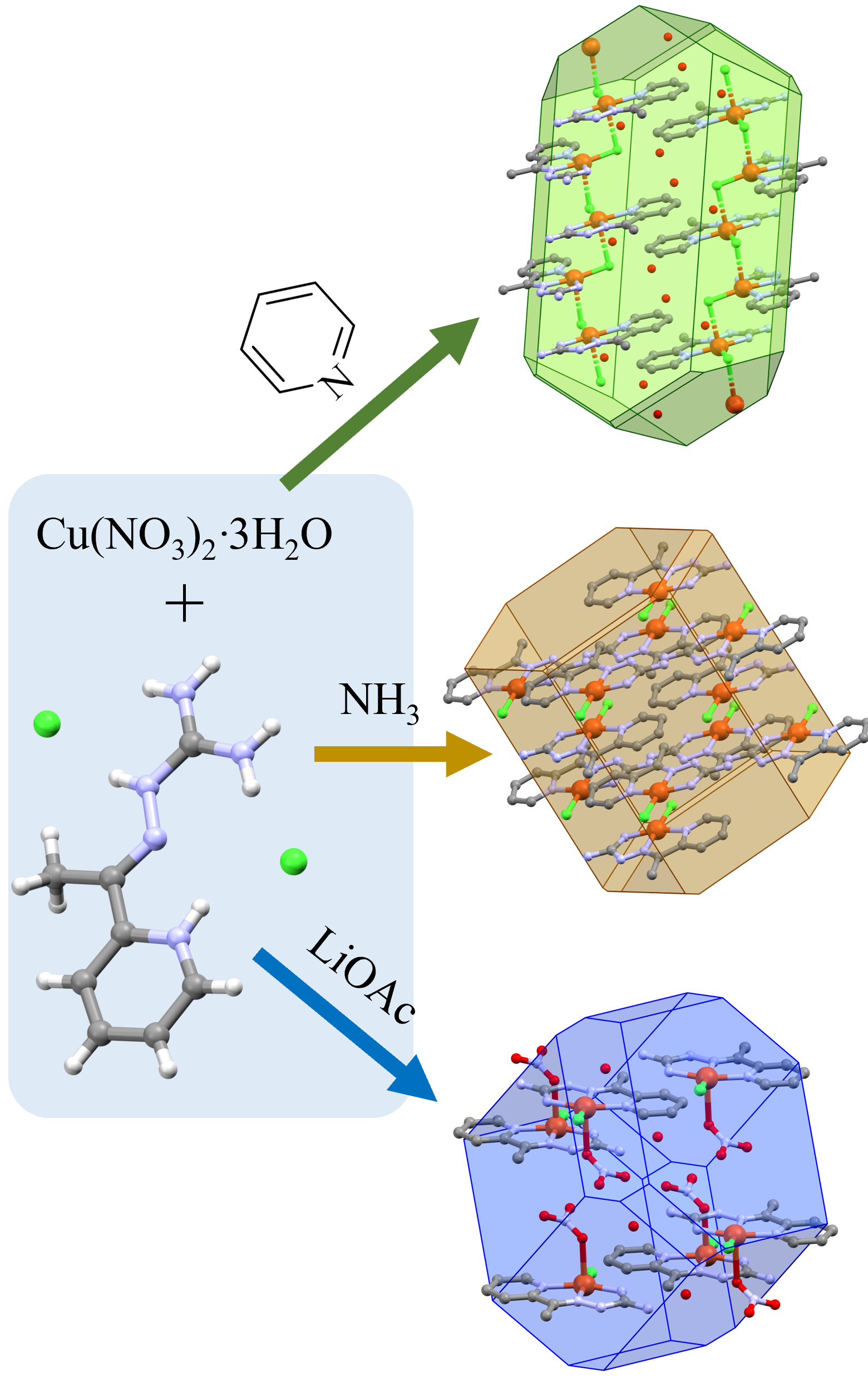
Aminoguanidine derivatives are the focus of research because of their various biological activities, such as antiviral, antibacterial, analgesic, antioxidant and anticancer. Their complexes with different metals are also examined and many of them show significant biological activity, too. Besides, some of the complexes show good photoluminescent properties and are used for the preparation of photoelectronic devices. Therefore, the synthesis, physicochemical, structural and thermal characterization of the complexes of 2-acetylpyridine-aminoguanidine (L) with copper (II) are described here. Under different reaction conditions, Cu(II) with L gives three complexes of different compositions. By varying the strength of basicity of the deprotonating agent used, it was proven here that the Schiff base given here could be coordinated in neutral or monoanionic form. In the presence of pyridine, a coordination polymer is obtained, while in the presence of ammonia/lithium acetate two different monomeric complexes were crystallised. Their physicochemical and thermal properties, as well as molecular and crystal structure, are determined.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-47/2023-01/200125 -
Javna Agencija za Raziskovalno Dejavnost RS
Grant numbers Slovenian–Serbian joint co-operation project
References
O. Dömötör, N. V. May, G. T. Gál, G. Spengler, A. Dobrova, V. B. Arion, É. A. Enyedy, Molecules 27 (2022) 2044 (https://doi.org/10.3390/MOLECULES27072044/S1)
J. Vojtaššák, J. Čársky, L. Danišovič, D. Böhmer, M. Blaško, T. Braxatorisová, Toxicol. in Vitro 20 (2006) 868 (https://doi.org/10.1016/j.tiv.2005.12.009)
C. Watala, M. Dobaczewski, P. Kazmierczak, J. Gebicki, M. Nocun, I. Zitnanova, O. Ulicna, Z. Durackova, I. Waczulíková, J. Carsky, S. Chlopicki, Vascul. Pharmacol. 51 (2009) 275 (https://doi.org/10.1016/j.vph.2009.07.002)
Yu. M. Chumakov, V. I. Tsapkov, G. Bocelli, B. Ya. Antosyak, S. G. Shova, A. P. Gulea, Crystallogr. Rep. 51 (2006) 60 (https://doi.org/10.1134/S1063774506010123)
M. S. More, P. G. Joshi, Y. K. Mishra, P. K. Khanna, Mater. Today Chem. 14 (2019) 100195 (https://doi.org/10.1016/j.mtchem.2019.100195)
C. Boulechfar, H. Ferkous, A. Delimi, A. Djedouani, A. Kahlouche, A. Boublia, A. S. Darwish, T. Lemaoui, R. Verma, Y. Benguerba, Inorg. Chem. Commun. 150 (2023) 110451 (https://doi.org/10.1016/j.inoche.2023.110451)
S. S. Chourasiya, D. Kathuria, S. S. Nikam, A. Ramakrishnan, S. Khullar, S. K. Mandal, A. K. Chakraborti, P. V. Bharatam, J. Org. Chem. 81 (2016) 7574 (https://doi.org/10.1021/acs.joc.6b01258)
M. G. Jelić, D. G. Georgiadou, M. M. Radanović, N. Ž. Romčević, K. P. Giannakopoulos, V. M. Leovac, L. F. Nađ, L. S. Vojinović-Ješić, Opt. Quantum Electron. 48 (2016) 276 (https://doi.org/10.1007/s11082-016-0547-5)
V. M. Leovac, M. D. Joksović, V. Divjaković, L. S. Jovanović, Ž. Šaranović, A. Pevec, J. Inorg. Biochem. 101 (2007) 1094 (https://doi.org/10.1016/j.jinorgbio.2007.04.004)
L. S. Vojinović-Ješić, M. M. Radanović, M. V. Rodić, V. Živković-Radovanović, L. S. Jovanović, V. M. Leovac, Polyhedron 117 (2016) 526 (https://doi.org/10.1016/J.POLY.2016.06.032)
M. M. Radanović, M. V. Rodić, L. S. Vojinović-Ješić, S. Armaković, S. J. Armaković, V. M. Leovac, Inorg. Chim. Acta 473 (2018) 160 (https://doi.org/10.1016/J.ICA.2017.12.038)
M. Radanovic, S. Novakovic, M. Rodic, L. Vojinovic-Jesic, C. Janiak, V. Leovac, J. Serb. Chem. Soc. 87 (2022) 1259 (https://doi.org/10.2298/JSC220613072R)
M. M. Radanović, L. S. Vojinović-Ješić, M. G. Jelić, E. Sakellis, B. Barta Holló, V. M. Leovac, M. V. Rodić, Inorganics (Basel) 10 (2022) 147 (https://doi.org/10.3390/inorganics10100147)
M. M. Radanović, M. V. Rodić, L. S. Vojinović-Ješić, S. Armaković, S. J. Armaković, V. M. Leovac, Inorg. Chim. Acta 473 (2018) 160 (https://doi.org/10.1016/j.ica.2017.12.038)
G. M. Sheldrick, Acta Crystallogr. A Found. Adv. 71 (2015) 3 (https://doi.org/10.1107/S2053273314026370)
G. M. Sheldrick, Acta Crystallogr., C 71 (2015) 3 (https://doi.org/10.1107/S2053229614024218)
A. L. Spek, Acta Crystallogr., D 65 (2009) 148 (https://doi.org/10.1107/S090744490804362X)
C. R. Groom, I. J. Bruno, M. P. Lightfoot, S. C. Ward, Acta Crystallogr., B 72 (2016) 171 (https://doi.org/10.1107/S2052520616003954)
I. J. Bruno, J. C. Cole, M. Kessler, J. Luo, W. D. S. Momerwell, L. H. Purkis, B. R. Smith, R. Taylor, R. I. Cooper, S. E. Harris, A. G. Orpen, J. Chem. Inf. Comput. Sci. 44 (2004) 2133 (https://doi.org/10.1021/CI049780B)
C. F. MacRae, I. Sovago, S. J. Cottrell, P. T. A. Galek, P. McCabe, E. Pidcock, M. Platings, G. P. Shields, J. S. Stevens, M. Towler, P. A. Wood, Urn:Issn:1600-5767 53 (2020) 226 (https://doi.org/10.1107/S1600576719014092)
K. Nakamoto, Infrared and Raman spectra of inorganic and coordination compounds. Part B, Applications in coordination, organometallic, and bioinorganic chemistry, Wiley, New York, 2009 (ISBN 978-0-471-74339-2)
A. W. Addison, T. N. Rao, J. Reedijk, J. van Rijn, G. C. Verschoor, J. Chem. Soc., Dalton Trans. 0 (1984) 1349 (https://doi.org/10.1039/DT9840001349)
L. Yang, D. R. Powell, R. P. Houser, J. Chem. Soc., Dalton Trans. (2007) 955 (https://doi.org/10.1039/b617136b)
NIST Chemistry WebBook, https://webbook.nist.gov/chemistry/ (accessed June 30, 2023).