Introducing a novel crystal form of pyruvic acid thiosemicarbazone and its sodium salt Scientific paper
Main Article Content
Abstract
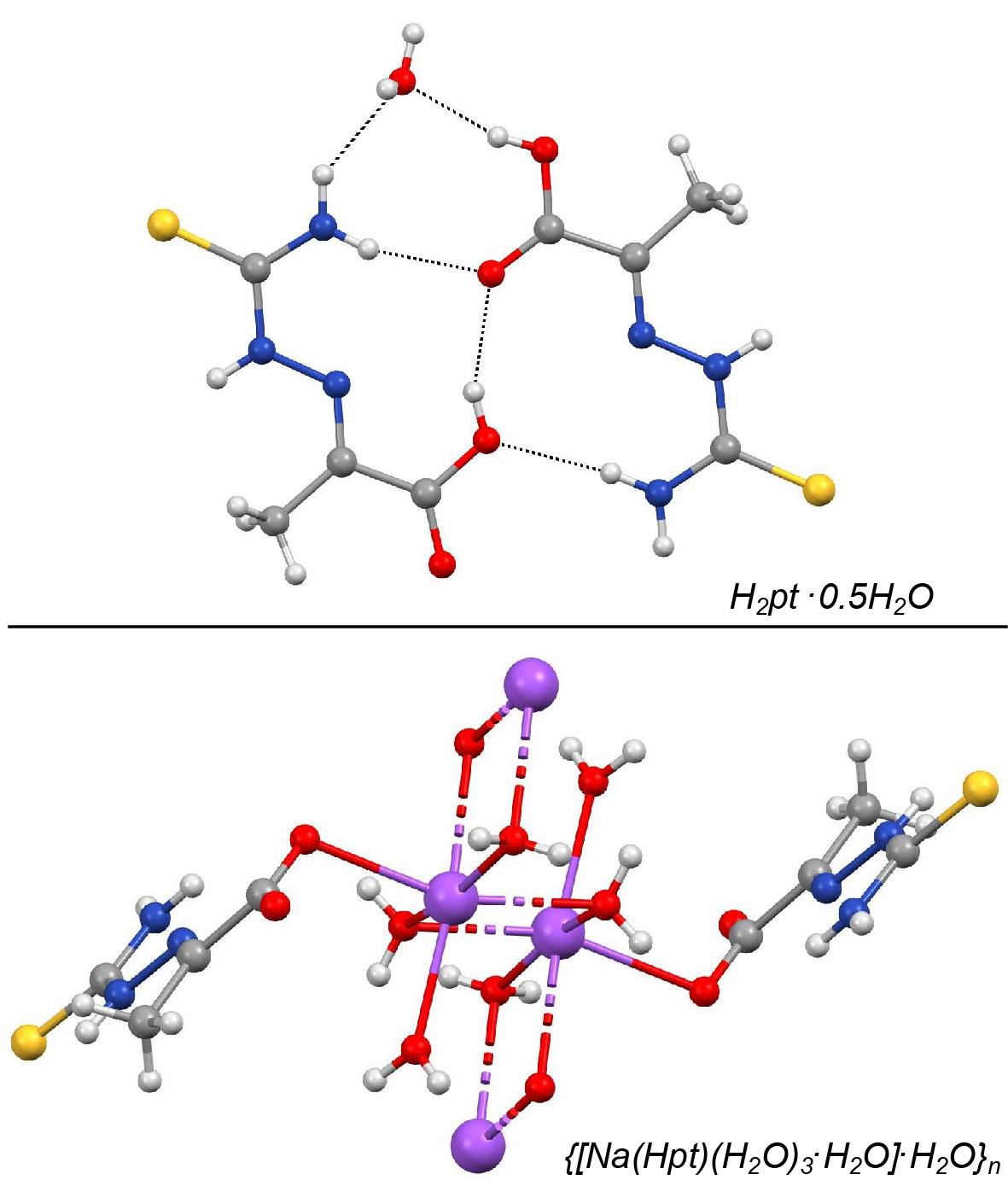
The reaction of thiosemicarbazide and sodium pyruvate has been thoroughly studied and the novel crystal form of pyruvic acid thiosemicarbazone (H2pt) and its sodium salt were obtained. Compounds were characterized by IR spectra, melting points, elemental analysis and conductometric measurements, as well as single-crystal X-ray analysis. A detailed comparative analysis of crystal structures of these compounds is given, as well as comparison with some of the earlier known complexes containing H2pt. The two novel crystal structures exhibit notably different hydrogen bonding patterns, mutually and in comparison with previously reported crystal form of H2pt. All crystal structures are stabilized by extensive network of N–H...O, O–H...O and N–H...S hydrogen bonds. The cyclic hydrogen bonding motif involving the thioureido moieties of the ligand is the only one which repeats in each structure.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-66/2024-03/200017;451-03-66/2024-03/200125;451-03-65/2024-03/200125
References
A. Gómez Quiroga, C. Navarro Ranninger, Coord. Chem. Rev. 248 (2004) 119 (https://doi.org/10.1016/j.cct.2003.11.004)
G. L. Parrilha, R. G. dos Santos, H. Beraldo, Coord. Chem. Rev. 458 (2022) 214418 (https://doi.org/10.1016/j.ccr.2022.214418)
H. Beraldo, D. Gambino, Mini-Rev. Med. Chem. 4 (2004) 31 (https://doi.org/10.2174/1389557043487484)
D.S. Kalinowski D. R.Richardson, Pharmacol. Rev. 57 (2005) 547 (https://doi.org/10.1124/pr.57.4.2)
A. Mushtaq, P. Wu, M. M. Naseer, Pharmacol. Ther. 254 (2024) 108579 (https://doi.org/10.1016/j.pharmthera.2023.108579)
O. Özbek, C. Berkel, Polyhedron 238 (2023) 116426 (https://doi.org/10.1016/j.poly.2023.116426)
L. Feng, W. Shi, J. Ma, Y. Chen, F. Kui, Y. Hui, Z. Xie, Sens. Actuators, B 237 (2016) 563 (https://doi.org/10.1016/j.snb.2016.06.129)
R. Basri, N. Ahmed, M. Khalid, M. Usman Khan, M. Abdullah, A. Syed, A. M. Elgorban, S. S.Al-Rejaie, A. A. C. Braga, Z. Shafq, Sci. Rep. 12 (2022) 4927 (https://doi.org/10.1038/s41598-022-08860-3)
E. Khamis, M. A. Ameer, N. M. AlAndis, G. Al-Senani, Corrosion 56 (2000) 127 (https://doi.org/10.5006/1.3280528)
Q. A. Jawad, D. S. Zinad, R. D. Salim,A. A Al-Amiery,T. S. Gaaz,M. S. Takriff, A. A. H. Kadhum, Coatings 9 (2019) 729 (https://doi.org/10.3390/coatings9110729)
T. S. Lobana, R. Sharma, G. Bawa, S. Khanna, Coord. Chem. Rev. 253 (2009) 977 (https://doi.org/10.1016/j.ccr.2008.07.004)
J.S. Casas, M.S. Garcia-Tasende, J. Sordo, Coord. Chem. Rev. 209 (2000) 197 (https://doi.org/10.1016/S0010-8545(00)00363-5)
D. X. West, S. B. Padhye, P. B. Sonawane, Struct. Bond. 76 (1991) 1 (https://doi.org/10.1007/3-540-53499-7_1)
J. Haribabu, K. Jeyalakshmi, Y. Arun, N. S. P. Bhuvanesh, P. T. Perumal, R. Karvembu, RSC Adv. 5 (2015) 46031 (https://doi.org/10.1039/C5RA04498G)
L. R. P. de Siqueira, P. A. T. de Moraes Gomes, L. P. de Lima Ferreira, M. J. B. de Melo Rêgo, A. C. L. Leite, Eur. J. Med. Chem. 170 (2019) 237 (https://doi.org/10.1016/j.ejmech.2019.03.024)
H. Dong, J. Liu, X. Liu, Y. Yu, S. Cao, Bioorg. Chem. 75 (2017) 106 (https://doi.org/10.1016/j.bioorg.2017.07.002)
J. Wiecek, V. Dokorou, Z. Ciunik, D. Kovala-Demertzi, Polyhedron 28 (2009) 3298 (https://doi.org/10.1016/j.poly.2009.05.012)
M. B. Ferrari, F. Bisceglie, G. Pelosi, P. Tarasconi, R. Albertini, S. Pinelli, J. Inorg. Bioch. 87 (2001) 137 (https://doi.org/10.1016/S0162-0134(01)00321-X)
I. Graur,T. Bespalova, V. Graur, V. Tsapkov, O. Garbuz, E. Melnic, P. Bourosh, A. Gulea, J. Chem. Res. 47 (2023) 6 (https://doi.org/10.1177/17475198231216422)
T.A. Yousef, G.A. El-Reash, O.A. El-Gammal, R. A. Bedier. J Mol Struct. 1035 (2013) 307 (https://doi.org/10.1016/j.molstruc.2012.10.058)
N. I. Dodoff, D. Kovala-Demertzi, M. Kubiak, J. Kuduk-Jaworska, A. Kochel, G. A. Gorneva, Z. Naturforsch., B 61 (2006) 1110 (https://doi.org/10.1515/znb-2006-0909)
B. Ya.Antosyak, V. N. Biyushkin, L. F. Chapurina, T. I. Malinovsky, Dokl. Akad. Nauk SSSR 327 (1992) 219
C. R. Groom, I. J. Bruno, M. P. Lightfoot, S. C. Ward, Acta Cryst., B 72 (2016) 171. (https://dx.doi.org/10.1107/S2052520616003954)
Rigaku Oxford Diffraction, CrysAlisPro Software system, version 1.171.42, Rigaku Corporation, Wroclaw, 2022
G. M. Sheldrick, Acta Cryst., C 71 (2015) 3 (https://doi.org/10.1107/S2053229614024218)
C. F. Macrae, I. Sovago, S. J. Cottrell, P. T. Galek, P. McCabe, E. Pidcock, P. A. Wood, J. Appl. Cryst. 53 (2020) 226 (https://doi.org/10.1107/S1600576719014092)
P. R. Spackman, M. J. Turner, J. J. McKinnon, S. K. Wolff, D. J. Grimwood, D. Jayatilaka, M. A. Spackman, J. Appl. Cryst. 54 (2021) 1006 (https://doi.org/10.1107/S1600576721002910)
G. L. Sawhney, J. S. Baijal, S. Chandra, K. B. Pandeya, Acta Chim. Acad. Sci. Hung. 108 (1981) 325
M. D. Timken, S. R. Wilson, D. N. Hendrickson, Inorg. Chem. 24 (1985) 3450 (https://dx.doi.org/10.1021/ic00215a030)
M. Belicchi Ferrari, Giovanna Gasparri Fava, G. Pelosi, P. Tarasconi, Polyhedron 19 (2000) 1895 (https://doi.org/10.1016/S0277-5387(00)00454-X)
M C Etter, J C MacDonald, J Bernstein, Acta Cryst., B 46 (1990) 256 (https://doi.org/10.1107/S0108768189012929)
S. B. Novaković, B. Fraisse, G. A. Bogdanović, A. Spasojevic-de Biré, Cryst. Growth Des. 17 (2007) 2993 (https://doi.org/10.1021/cg060497).