Synthesis and structural analysis of tetranuclear Zn(II) complex with 2,3-dihydroxybenzaldehyde-aminoguanidine Scientific paper
Main Article Content
Abstract
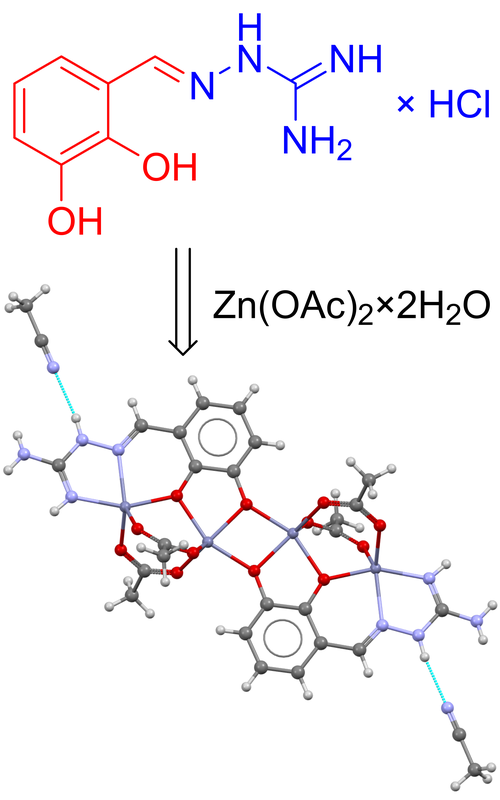
Here we report a new Schiff base of aminoguanidine and 2,3-dihydroxybenzaldehyde (H2L) and its physicochemical characterization, along with an investigation into its coordination affinities towards zinc. By reacting zinc acetate with the chloride salt of the ligand in the MeCN–H2O solution, yellow single-crystals of tetranuclear, centrosymmetric complex, with the formula [Zn2(µ-L)(µ-OAc)2]2∙2MeCN, were obtained. The complex was characterized by IR spectroscopy, conductometry, elemental analysis, and single-crystal X-ray diffraction analysis. Notably, both nitrogen atoms of the aminoguanidine residue coordinate to the same zinc atom, while both deprotonated phenyl oxygen atoms achieve bridging coordination. Furthermore, two acetate anions bridge adjacent zinc atoms in addition to the Schiff base anion. Meaningful insights into the hierarchy and significance of intermolecular interactions within the crystal structure were gained by estimating the energies using the CrystalExplorer model. The calculations revealed that the crystal structure can be classified as a layer type, with notably stronger interactions occurring along the [001] and [011] directions.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-47/2023-01/200125
References
D. Aggoun, Z. Messasma, B. Bouzerafa, R. Berenguer, E. Morallon, Y. Ouennoughi, A. Ourari, J. Mol. Struct. 1231 (2021) 129923 (https://doi.org/10.1016/J.MOLSTRUC.2021.129923)
A. Kajal, S. Bala, S. Kamboj, N. Sharma, V. Saini, Journal of Catalysts 2013 (2013) 1 (https://doi.org/10.1155/2013/893512)
Saswati, M. Mohanty, A. Banerjee, S. Biswal, A. Horn, G. Schenk, K. Brzezinski, E. Sinn, H. Reuter, R. Dinda, J. Inorg. Biochem. 203 (2020) 110908 (https://doi.org/10.1016/J.JINORGBIO.2019.110908)
M. Barwiolek, D. Jankowska, M. Chorobinski, A. Kaczmarek-Kędziera, I. Łakomska, S. Wojtulewski, T. M. Muzioł, RSC Adv. 11 (2021) 24515 (https://doi.org/10.1039/D1RA03096E)
P. Mahadevi, S. Sumathi, Synth. Commun. 50 (2020) 2237 (https://doi.org/10.1080/00397911.2020.1748200)
H. Kargar, M. Fallah-Mehrjardi, R. Behjatmanesh-Ardakani, H. A. Rudbari, A. A. Ardakani, S. Sedighi-Khavidak, K. S. Munawar, M. Ashfaq, M. N. Tahir, Inorg. Chim. Acta 530 (2022) 120677 (https://doi.org/10.1016/J.ICA.2021.120677)
M. Martínez Belmonte, E. C. Escudero-Adán, E. Martin, A. W. Kleij, Dalton Transactions 41 (2012) 5193 (https://doi.org/10.1039/C2DT30201B)
B. B. Tang, H. Ma, G. Z. Li, Y. B. Wang, G. Anwar, R. Shi, H. Li, CrystEngComm 15 (2013) 8069 (https://doi.org/10.1039/C3CE41034J)
R. Biswas, C. Diaz, A. Ghosh, Polyhedron 56 (2013) 172 (https://doi.org/10.1016/J.POLY.2013.03.046)
J. Adhikary, P. Chakraborty, S. Samanta, E. Zangrando, S. Ghosh, D. Das, Spectrochim. Acta A Mol Biomol Spectrosc 178 (2017) 114 (https://doi.org/10.1016/j.saa.2017.01.041)
B. Babic, M. Romcevic, M. Gilic, J. Trajic, M. M. Radanović, L. S. Vojinović-Ješić, M. V. Rodić, N. Romcevic, Opt. Mater. (Amst) 136 (2023) 113445 (https://doi.org/10.1016/J.OPTMAT.2023.113445)
Rigaku Oxford Diffraction, CrysAlisPro Software system, Rigaku Corporation, Oxford, 2022
G. M. Sheldrick, Acta Cryst., A 71 (2015) 3 (https://doi.org/10.1107/S2053273314026370)
G. M. Sheldrick, Acta Cryst. C Struct. Chem. 71 (2015) 3 (https://doi.org/10.1107/S2053229614024218)
C. B. Hübschle, G. M. Sheldrick, B. Dittrich, J. Appl. Cryst. 44 (2011) 1281 (https://doi.org/10.1107/S0021889811043202)
A. L. Spek, Acta Cryst. D Biol Cryst. 65 (2009) 148 (https://doi.org/10.1107/S090744490804362X)
C. R. Groom, I. J. Bruno, M. P. Lightfoot, S. C. Ward, Acta Cryst., B 72 (2016) 171 (https://doi.org/10.1107/S2052520616003954)
I. J. Bruno, J. C. Cole, M. Kessler, J. Luo, W. D. S. Momerwell, L. H. Purkis, B. R. Smith, R. Taylor, R. I. Cooper, S. E. Harris, A. G. Orpen, J. Chem. Inf. Comp. Sci. 44 (2004) 2133 (https://doi.org/10.1021/CI049780B)
C. F. MacRae, I. Sovago, S. J. Cottrell, P. T. A. Galek, P. McCabe, E. Pidcock, M. Platings, G. P. Shields, J. S. Stevens, M. Towler, P. A. Wood, J. Appl. Cryst. 53 (2020) 226 (https://doi.org/10.1107/S1600576719014092)
P. R. Spackman, M. J. Turner, J. J. McKinnon, S. K. Wolff, D. J. Grimwood, D. Jayatilaka, M. A. Spackman, J. Appl. Cryst. 54 (2021) 1006 (https://doi.org/10.1107/S1600576721002910)
D. Jayatilaka, D. J. Grimwood, in Computational Science — ICCS 2003. ICCS 2003. Lecture Notes in Computer Science, P. M. A. Sloot, D. Abramson, A. V. Bogdanov, Y. E. Gorbachev, J. J. Z. A. Y. Dongarra, Eds., Springer, Berlin, 2003
S. P. Thomas, P. R. Spackman, D. Jayatilaka, M. A. Spackman, J. Chem. Theor. Comput. 14 (2018) 1614 (https://doi.org/https://doi.org/10.1021/acs.jctc.7b01200)
W. K. Dong, J. Zhang, Y. Zhang, N. Li, Inorg. Chim. Acta 444 (2016) 95 (https://doi.org/10.1016/J.ICA.2016.01.034)
K. Nakamoto, Infrared and Raman spectra of inorganic and coordination compounds. Part B, Applications in coordination, organometallic, and bioinorganic chemistry, Wiley, New York, 2009
A. W. Addison, T. N. Rao, J. Reedijk, J. van Rijn, G. C. Verschoor, J. Chem. Soc., Dalton Trans. (1984) 1349 (https://doi.org/10.1039/DT9840001349)
R. R. Holmes, J. A. Deiters, J. Am. Chem. Soc. 99 (1977) 3318 (https://doi.org/10.1021/ja00452a021)
R. R. Holmes, Acc. Chem. Res. 12 (1979) 257 (https://doi.org/10.1021/ar50139a006)
R. R. Holmes, in Progress in Inorganic Chemistry, S. J. Lippard, Ed., Wiley, New York, 2007, pp. 119–235 (https://doi.org/10.1002/9780470166338.ch2)
M. Pinsky, D. Avnir, Inorg. Chem. 37 (1998) 5575 (https://doi.org/doi.org/10.1021/ic9804925)
D. Casanova, J. Cirera, M. Llunell, P. Alemany, D. Avnir, S. Alvarez, J. Am. Chem. Soc. 126 (2004) 1755 (https://doi.org/10.1021/ja036479n)
Lj. S. Vojinović-Ješić, M. M. Radanović, Coordination chemistry of aminoguanidine and its Schiff bases, Faculty of Sciences, Novi Sad, 2017 (https://www.pmf.uns.ac.rs/wp-content/uploads/2016/04/vojinovicjesic_radanovic_koordinaciona_hemija_aminogvanidina.pdf) (in Serbian)
M. M. Belmonte, E. C. Escudero-Adán, E. Martin, A. W. Kleij, Dalton Trans. 41 (2012) 5193 (https://doi.org/10.1039/C2DT30201B)
S. P. Thomas, P. R. Spackman, D. Jayatilaka, M. A. Spackman, J. Chem. Theory Comput. 14 (2018) 1614 (https://doi.org/10.1021/ACS.JCTC.7B01200)
M. C. Etter, J. C. MacDonald, J. Bernstein, Acta Cryst. B Struct. Sci. Cryst. Eng. Mater. 46 (1990) 256 (https://doi.org/10.1107/S0108768189012929).