Zn(II) complex with pyridine based 1,3-selenazolyl-hydrazone: Synthesis, structural characterization and DFT study Scientific paper
Main Article Content
Abstract
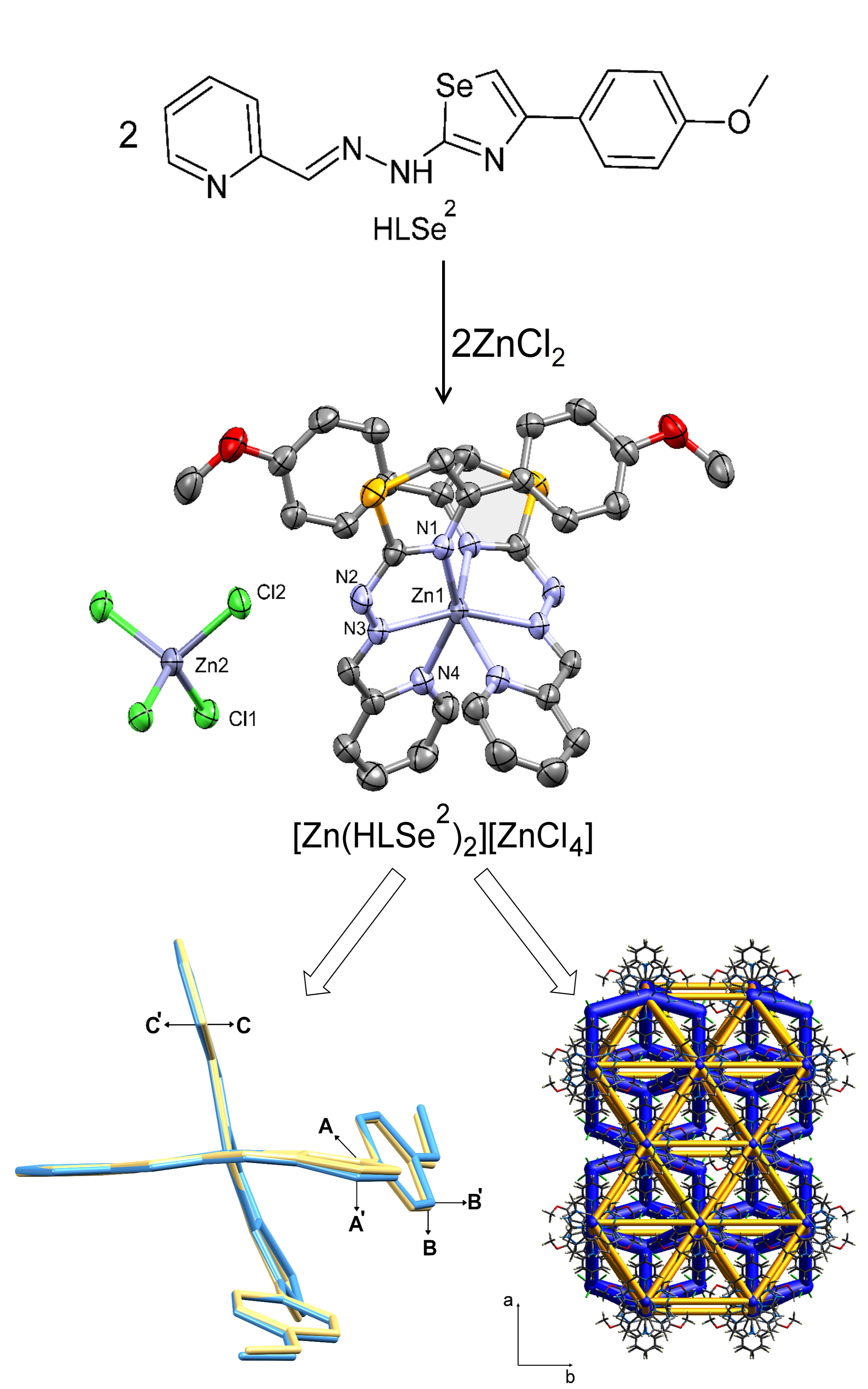
An octahedral complex of Zn(II) with a ligand from a class of pyridine-based 1,3-selenazolyl-hydrazones was synthesized and characterized by IR and NMR spectroscopy and single crystal X-ray diffraction analysis. The purity of the complex was confirmed by elemental analysis. Two ligands are coordinated in the neutral NNN-tridentate form forming a complex cation, while the positive charge is neutralized by [ZnCl4]2-. Complex crystallizes in monoclinic C2/c space group with the Zn atoms situated in a special position. The packing features of the novel complex were analyzed using Hirshfeld surfaces, construction of 2D pseudosymmetric plot and DFT quantum mechanical calculations and compared with the previously published sulfur-based isostere. The key difference in the structures, imposed by replacement of sulfur with selenium, were identified.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja,Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-47/2023-01/200168;451-03-47/2023-01/200288;451-03-47/2023-01/200116
References
Y. Su, I. Cockerill, Y. Wang, Y. X. Qin, L. Chang, Y. Zheng, D. Zhu, Trends Biotechnol. 37 (2019) 428 (https://doi.org/10.1016/j.tibtech.2018.10.009)
T. Zhang, X. Li, Y. Qiu, P. Su, W. Xu, H. Zhong, H. Zhang, J. Catal. 357 (2018) 154 (https://doi.org/10.1016/j.jcat.2017.11.003)
D. Drozd, K. Szczubiałka, Ł. Łapok, M. Skiba, H. Patel, S. M. Gorun, M. Nowakowska, Appl. Catal., B 125 (2012) 35 (https://doi.org/10.1016/j.apcatb.2012.05.021)
J. B. Araškov, A. Višnjevac, J. Popović, V. Blagojević, H. S. Fernandes, S. F. Sousa, I. Novaković, J. M. Padrón, B. B. Holló, M. Monge, M. Rodríguez-Castillo, J. M. López-
-De-Luzuriaga, N. R. Filipović, T. R. Todorović, CrystEngComm 24 (2022) 5194 (https://doi.org/10.1039/d2ce00443g)
Z. Li, A. Dellali, J. Malik, M. Motevalli, R. M. Nix, T. Olukoya, Y. Peng, H. Ye, W. P. Gillin, I. Hernández, P. B. Wyatt, Inorg. Chem. 52 (2013) 1379 (https://doi.org/10.1021/ic302063u)
S. D. Han, N. N. Rajput, X. Qu, B. Pan, M. He, M. S. Ferrandon, C. Liao, K. A. Persson, A. K. Burrell, ACS Appl. Mater. Interfaces 8 (2016) 3021 (https://doi.org/10.1021/acsami.5b10024)
C. R. Groom, I. J. Bruno, M. P. Lightfoot, S. C. Ward, Acta Crystallogr., B 72 (2016) 171 (https://doi.org/10.1107/S2052520616003954)
P. Ristić, V. Blagojević, G. Janjić, M. Rodić, P. Vulić, M. Donnard, M. Gulea, A. Chylewska, M. Makowski, T. Todorović, N. Filipović, Cryst. Growth Des. 20 (2020) 3018 (https://doi.org/10.1021/acs.cgd.9b01661)
N. J. Williams, W. Gan, J. H. Reibenspies, R. D. Hancock, Inorg. Chem. 48 (2009) 1407 (https://doi.org/10.1021/ic801403s)
Z. Xun-Zhong, F. An-Sheng, Z. Fu-Ran, L. Min-Cheng, L. Yan-Zhi, M. Meng, L. Yu, Bioinorg. Chem. Appl. 2020 (2020) 1 (https://doi.org/10.1155/2020/8852470)
X. Zou, Y. Liao, C. Yang, A. Feng, X. Xu, H. Jiang, Y. Li, J. Coord. Chem. 74 (2021) 1009 (https://doi.org/10.1080/00958972.2020.1869952)
J. B. Araškov, M. Nikolić, S. Armaković, S. Armaković, M. Rodić, A. Višnjevac, J. M. Padrón, T. R. Todorović, N. R. Filipović, J. Mol. Struct. 1240 (2021) 130512 (https://doi.org/10.1016/j.molstruc.2021.130512)
L. K. Durgeswari, R. K. Ganta, Y. L. N. Murthy, Russ. J. Org. Chem. 57 (2021) 1552 (https://doi.org/10.1134/s1070428021090232)
A. A. Alfi, A. Alharbi, J. Qurban, M. M. Abualnaja, H. M. Abumelha, F. A. Saad, N. M. El-Metwaly, J. Mol. Struct. 1267 (2022) 133582 (https://doi.org/10.1016/j.molstruc.2022.133582)
V. A. Adole, R. A. More, B. S. Jagdale, T. B. Pawar, S. S. Chobe, ChemistrySelect 5 (2020) 2778 (https://doi.org/10.1002/slct.201904609)
S. Abu-Melha, Pigment Resin Technol. 48 (2019) 375 (https://doi.org/10.1108/PRT-09-2018-0102)
G. S. Masaret, ChemistrySelect 6 (2021) 974 (https://doi.org/10.1002/slct.202004304)
M. S. Shah, M. M. Rahman, M. D. Islam, A. Al-Macktuf, J. U. Ahmed, H. Nishino, M. A. Haque, J. Mol. Struct. 1248 (2022) 131465 (https://doi.org/10.1016/j.molstruc.2021.131465)
O. A. El-Khouly, M. A. Henen, M. A. A. El-Sayed, M. I. Shabaan, S. M. El-Messery, Bioorganic Med. Chem. 31 (2021) 115976 (https://doi.org/10.1016/j.bmc.2020.115976)
R. M. Kassab, S. M. Gomha, S. A. Al-Hussain, A. S. Abo Dena, M. M. Abdel-Aziz, M. E. A. Zaki, Z. A. Muhammad, Arab. J. Chem. 14 (2021) 103396 (https://doi.org/10.1016/j.arabjc.2021.103396)
S. Abu-Melha, Arab. J. Chem. 15 (2022) 103898 (https://doi.org/10.1016/j.arabjc.2022.103898)
A. Y. Alzahrani, Y. A. Ammar, M. Abu-Elghait, M. A. Salem, M. A. Assiri, T. E. Ali, A. Ragab, Bioorg. Chem. 119 (2022) 105571 (https://doi.org/10.1016/j.bioorg.2021.105571)
A. Pricopie, I. Ionuţ, G. Marc, A. Arseniu, L. Vlase, A. Grozav, L. Găină, D. C. Vodnar, A. Pîrnău, B. Tiperciuc, O. Oniga, Molecules 24 (2019) 3435 (https://doi.org/10.3390/molecules24193435)
N. J. C. Oliveira, I. N. S. Teixeira, P. O. Fernandes, G. C. Veríssimo, A. D. Valério, C. P. de S. Moreira, T. R. Freitas, A. C. V. Fonseca, A. de P. Sabino, S. Johann, V. G. Maltarollo, R. B. de Oliveira, J. Mol. Struct. 1267 (2022) (https://doi.org/10.1016/j.molstruc.2022.133573)
R. Gondru, S. Kanugala, S. Raj, C. Ganesh Kumar, M. Pasupuleti, J. Banothu, R. Bavantula, Bioorganic Med. Chem. Lett. 33 (2021) 127746 (https://doi.org/10.1016/j.bmcl.2020.127746)
S. Mor, S. Sindhu, S. Nagoria, M. Khatri, P. Garg, H. Sandhu, A. Kumar, J. Heterocycl. Chem. 56 (2019) 1622 (https://doi.org/10.1002/jhet.3548)
M. R. Shaaban, T. A. Farghaly, A. M. R. Alsaedi, Polycycl. Aromat. Compd. 42 (2022) 2521 (https://doi.org/10.1080/10406638.2020.1837887)
A. Pricopie, M. Focşan, I. Ionuţ, G. Marc, L. Vlase, L. Găină, D. C. Vodnar, E. Simon, G. Barta, A. Pîrnău, O. Oniga, Molecules 25 (2020) 1079 (https://doi.org/10.3390/molecules25051079)
S. S. Jadav, V. N. Badavath, R. Ganesan, N. M. Ganta, D. Besson, V. Jayaprakash, Anti-
-Infective Agents 18 (2018) 101 (https://doi.org/10.2174/2211352516666181016122537)
M. V. de O. Cardoso, G. B. de Oliveira Filho, L. R. P. de Siqueira, J. W. P. Espíndola, E. B. da Silva, A. P. de O. Mendes, V. R. A. Pereira, M. C. A. B. de Castro, R. S. Ferreira, F. S. Villela, F. M. R. da Costa, C. S. Meira, D. R. M. Moreira, M. B. P. Soares, A. C. L. Leite, Eur. J. Med. Chem. 180 (2019) 191 (https://doi.org/10.1016/j.ejmech.2019.07.018)
A. Višnjevac, J. B. Araškov, M. Nikolić, Ž. Bojić-Trbojević, A. Pirković, D. Dekanski, D. Mitić, V. Blagojević, N. R. Filipović, T. R. Todorović, J. Mol. Struct. 1281 (2023) 135193 (https://doi.org/10.1016/j.molstruc.2023.135193)
D. Radomska, R. Czarnomysy, D. Radomski, A. Bielawska, K. Bielawski, Nutrients 13 (2021) 1 (https://doi.org/10.3390/nu13051649)
D. Radomska, R. Czarnomysy, D. Radomski, K. Bielawski, Int. J. Mol. Sci. 22 (2021) 1 (https://doi.org/10.3390/ijms22031009)
N. R. Filipović, H. Elshaflu, S. Grubišić, L. S. Jovanović, M. Rodić, I. Novaković, A. Malešević, I. S. Djordjević, H. Li, N. Šojić, A. Marinković, T. R. Todorović, Dalt. Trans. 46 (2017) 2910 (https://doi.org/10.1039/c6dt04785h)
Agilent Technologies UK Ltd., CrysAlisPro Software system, 2014 (n.d.)
G. M. Sheldrick, Acta Crystallogr., C 71 (2015) 3 (https://doi.org/10.1107/S2053229614024218)
A. L. Spek, Acta Crystallogr., D 65 (2009) 148 (https://doi.org/10.1107/S090744490804362X)
C. F. MacRae, I. Sovago, S. J. Cottrell, P. T. A. Galek, P. McCabe, E. Pidcock, M. Platings, G. P. Shields, J. S. Stevens, M. Towler, P. A. Wood, J. Appl. Crystallogr. 53 (2020) 226 (https://doi.org/10.1107/S1600576719014092)
L. J. Farrugia, J. Appl. Crystallogr. 45 (2012) 849 (https://doi.org/10.1107/S0021889812029111)
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, H. Puschmann, J. Appl. Crystallogr. 42 (2009) 339 (https://doi.org/10.1107/S0021889808042726)
P. R. Spackman, M. J. Turner, J. J. McKinnon, S. K. Wolff, D. J. Grimwood, D. Jayatilaka, M. A. Spackman, J. Appl. Crystallogr. 54 (2021) 1006 (https://doi.org/10.1107/S1600576721002910)
Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford, CT, 2009
T. Lecklider, EE Eval. Eng. 50 (2011) 36 (https://go.gale.com/ps/i.do?p=AONE&u=anon~9e9d6b50&id=GALE|A272486022&v=2.1&it=r&sid=googleScholar&asid=467f45d4)
A. D. Becke, J. Chem. Phys. 104 (1996) 1040 (https://doi.org/10.1063/1.470829)
A. D. Becke, J. Chem. Phys. 96 (1992) 2155 (https://doi.org/10.1063/1.462066)
A. D. Becke, J. Chem. Phys. 98 (1993) 5648 (https://doi.org/10.1063/1.464913)
N. Godbout, D. R. Salahub, J. Andzelm, E. Wimmer, Can. J. Chem. 70 (1992) 560 (https://doi.org/10.1139/v92-079)
C. Sosa, J. Andzelm, B. C. Elkin, E. Wimmer, K. D. Dobbs, D. A. Dixon, J. Phys. Chem. 96 (1992) 6630 (https://doi.org/10.1021/j100195a022)
C. F. Mackenzie, P. R. Spackman, D. Jayatilaka, M. A. Spackman, IUCrJ 4 (2017) 575 (https://doi.org/10.1107/S205225251700848X).