Synthesis and in vitro study of redox properties of pyrrole and halogenated pyrrole derivatives Scientific paper
Main Article Content
Abstract
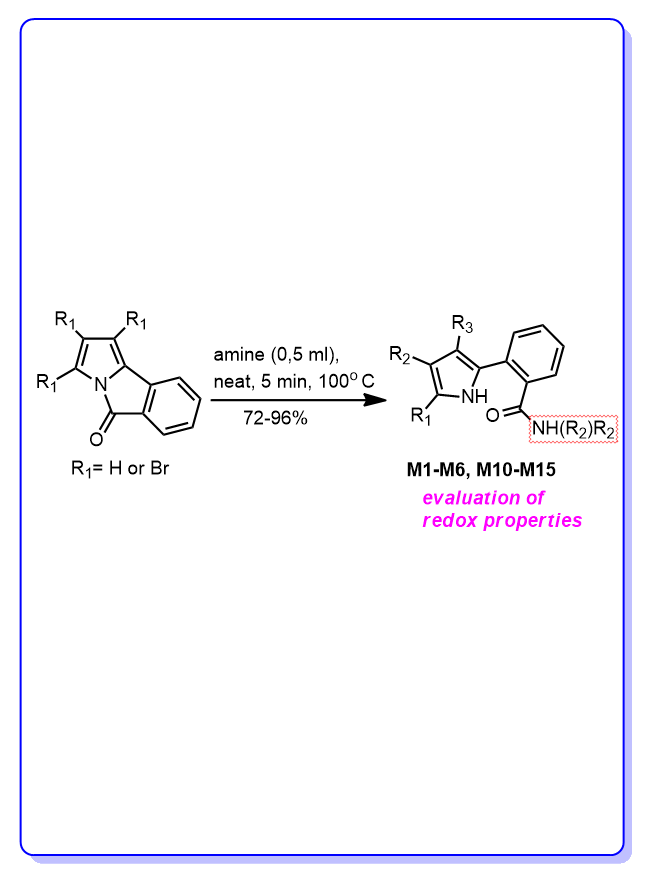
The redox balance plays a crucial role in maintaining biological processes under normal conditions. Antioxidants inhibit and reduce harmful oxidation processes, while pro-oxidants can act as anti-cancer agents by promoting ROS-mediated cell death. The aim of this study is to compare the redox properties of seven newly synthesised tribromopyrrole derivatives with three novel and four previously synthesized non-halogenated analogues in an in vitro model (in human serum) and with exogenously induced oxidative stress. The obtained values of their oxy scores (OS) were compared and the result showed that four non-halogenated pyrrole derivatives with secondary amide group M2, M10, M11 and M12 have lower OS values than Trolox, a water-soluble analogue of vitamin E with proven antioxidant properties. All four compounds show strong resistance to oxidative stress, which is reflected in the maintenance of negative OS values when exposed to exogenous oxidative stress using TBH in the reaction mixture. This capability to resist invading ROS should be expected also in an endogenous environment, where constant prooxidant production takes place at a low, homeostatic level, but even more so in pathological conditions. The tribrominated derivative M15 showed prooxidant activity with a significantly higher OS value than all other compounds tested. The comparison of the dose-response of Trolox and the five compounds with the lowest OS also shows that compounds M2, M7 and M10 have better antioxidant activity than Trolox.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-65/2024-03/ 200161;451-03-66/2024-03/ 200161
References
T. Finkel, J. Cell Biol. 194 (2011) 7 (https://doi.org/10.1083/jcb.201102095)
M. Valko, D. Leibfritz, J. Moncol, M. T. D. Cronin, M. Mazur, J. Telser, Int. J. Cell Biol. 39 (2007) 44 (https://doi.org/10.1016/j.biocel.2006.07.001)
S. Arfin, N. K. Jha, S. K. Jha, K. K. Kesari, J. Ruokolainen, S. Roychoudhury, B. Rathi, D. Kumar, Antioxidants 10 (2021) 642 (https://doi.org/10.3390/antiox10050642)
J. D. Hayes, A. T. Dinkova-Kostova, K. D. Tew, Cancer Cell 38 (2020) 167 (https://doi.org/10.1016/j.ccell.2020.06.001)
D. Trachootham, W. Lu, M. A. Ogasawara, N. R.-D. Valle, P. Huang, Antioxid. Redox. Signal. 10 (2008) 1343 (https://doi.org/10.1089/ars.2007.1957)
S. Toyokuni, Arch. Biochem. Biophys. 595 (2016) 46 (https://doi.org/10.1016/j.abb.2015.11.025)
S. Ahmad, O. Alam, Mohd. J. Naim, M. Shaquiquzzaman, M. M. Alam, M. Iqbal, Eur. J. Med. Chem. 157 (2018) 527 (https://doi.org/10.1016/j.ejmech.2018.08.002)
N. Jeelan Basha, S. M. Basavarajaiah, K. Shyamsunder, Mol. Divers 26 (2022) 2915 (https://doi.org/10.1007/s11030-022-10387-8)
R. H. Abd El-Hameed, A. I. Sayed, S. Mahmoud Ali, M. A. Mosa, Z. M. Khoder, S. S. Fatahala, J. Enzyme Inhib. Med. Chem. 36 (2021) 2183 (https://doaj.org/article/f1c9b4360a3c4baead207ed8f7344c44)
P. Rawat, R. N. Singh, A. Ranjan, A. Gautam, S. Trivedi, M. Kumar, J. Mol. Struct 1228 (2021) 129483 (https://doi.org/10.1016/j.molstruc.2020.129483)
A. R. Pandey, S. P. Singh, P. Joshi, K. S. Srivastav, S. Srivastava, K. Yadav, R. Chandra, A. C. Bisen, S. Agrawal, S. N. Sanap, R. S. Bhatta, R. Tripathi, M. K. Barthwal, K. V. Sashidhara, Eur. J. Med. Chem. 254 (2023) 115340 (https://doi.org/10.1016/j.ejmech.2023.115340)
J. A. Pfefferkorn, Y. Song, K.-L. Sun, S. R. Miller, B. K. Trivedi, C. Choi, R. J. Sorenson, L. D. Bratton, P. C. Unangst, S. D. Larsen, T.-J. Poel, X.-M. Cheng, C. Lee, N. Erasga, B. Auerbach, V. Askew, L. Dillon, J. C. Hanselman, Z. Lin, G. Lu, A. Robertson, K. Olsen, T. Mertz, C. Sekerke, A. Pavlovsky, M. S. Harris, G. Bainbridge, N. Caspers, H. Chen, M. Eberstadt, Bioorganic Med. Chem. Lett. 17 (2007) 4538 (https://doi.org/10.1016/j.bmcl.2007.05.096)
E. Mateev, M. Georgieva, A. Zlatkov, J. Pharm. Pharm. Sci. 25 (2022) 24 (https://doi.org/10.18433/jpps32417)
S. Cascioferro, M. Raimondi, M. Cusimano, D. Raffa, B. Maggio, G. Daidone, D. Schillaci, Molecules 20 (2015) 21658 (https://doi.org/10.3390/molecules201219797)
P. D. MacLean, E. E. Chapman, S. L. Dobrowolski, A. Thompson, L. R. C. Barclay, J. Org. Chem. 73 (2008) 6623 (https://doi.org/10.1021/jo8005073)
S. Matsugo, Y. Nakamura, Molecules 28 (2023) 2599 (https://pubmed.ncbi.nlm.nih.gov/36985566/)
G. Mallikarjuna Reddy, A. Camilo, J. Raul Garcia, Bioorg. Chem. 106 (2021) 104465 (https://doi.org/10.1016/j.bioorg.2020.104465)
A. Khalilpour, S. Asghari, Med. Chem. Res. 27 (2018) 15 (https://doi.org/10.1007/s00044-017-2041-4)
D. Tzankova, D. Aluani, M. Kondeva-Burdina, M. Georgieva, S. Vladimirova, L. Peikova, V. Tzankova, Pharm. Chem. J. 55 (2022) 1310 (https://doi.org/10.1007/s11094-022-02577-3)
J. D. Bhosale, A. R. Shirolkar, U. D. Pete, C. M. Zade, D. P. Mahajan, C. D. Hadole, S. D. Pawar, U. D. Patil, R. Dabur, R. S. Bendre, J. Pharm. Res. 7 (2013) 582 (https://doi.org/10.1016/j.jopr.2013.07.022)
M. Simic, J. Kotur-Stevuljevic, P. Jovanovic, M. Petkovic, M. Jovanovic, G. Tasic, V. Savic, J Serb Chem Soc 88 (2023) 589 (https://doi.org/10.2298/JSC221221017S)
S. Shetty, B. Kalluraya, Der Pharma Chem, 7 (2015) 26 (https://www.derpharmachemica.com/pharma-chemica/design-and-synthesis-of-hydrazone-incorporated-pyrazoles-and-triazoles-aspossible-antioxidants.pdf)
M. Petkovic, M. Jovanovic, P. Jovanovic, M. Simic, G. Tasic, V. Savic, Synth. 54 (2022) 2839 (https://doi.org/10.1055/a-2201-9951)
M. Simic, G. Tasic, P. Jovanovic, M. Petkovic, V. Savic, Org. Biomol. Chem. 16 (2018) 2125 (https://doi.org/10.1039/c8ob00260f)
M. Scipioni, G. Kay, I. Megson, P. Kong Thoo Lin, Eur. J. Med. Chem. 143 (2018) 745 (https://doi.org/10.1016/j.ejmech.2017.11.072)
O. Erel, Clin. Biochem. 38 (2005) 1103 (https://doi.org/10.1016/j.clinbiochem.2005.08.008)
J. Kotur-Stevuljevic, N. Bogavac-Stanojevic, Z. Jelic-Ivanovic, A. Stefanovic, T. Gojkovic, J. Joksic, M. Sopic, B. Gulan, J. Janac, S. Milosevic, Atherosclerosis 241 (2015) 192 (https://doi.org/10.1016/j.atherosclerosis.2015.05.016)
O. Erel, Clin. Biochem. 37 (2004) 277 (https://doi.org/10.1016/j.clinbiochem.2003.11.015)
D. H. Alamdari, K. Paletas, T. Pegiou, M. Sarigianni, C. Befani, G. Koliakos, Clin. Biochem. 40 (2007) 248 (https://doi.org/10.1016/j.clinbiochem.2006.10.017)
R. Sotler, B. Poljšak, R. Dahmane, T. Jukić, D. Pavan Jukić, C. Rotim, P. Trebše, A. Starc., ACC 58 (2019) 726 (https://doi.org/10.20471/acc.2019.58.04.20)
M. Cindrić, I. Sović, M. Mioč, L. Hok, I. Boček, P. Roškarić, K. Butković, I. Martin-Kleiner, K. Starčević, R. Vianello, M. Kralj, M. Hranjec, Antioxidants 8 (2019) 477 (https://doi.org/10.3390/antiox8100477)
A. Tai, T. Sawano, F. Yazama, H. Ito, Biochim. Biophys. Acta 1810 (2011) 170 (https://doi.org/10.1016/j.bbagen.2010.11.004).