New structure-based models for the prediction of normal boiling point temperature of ternary azeotropes Scientific paper
Main Article Content
Abstract
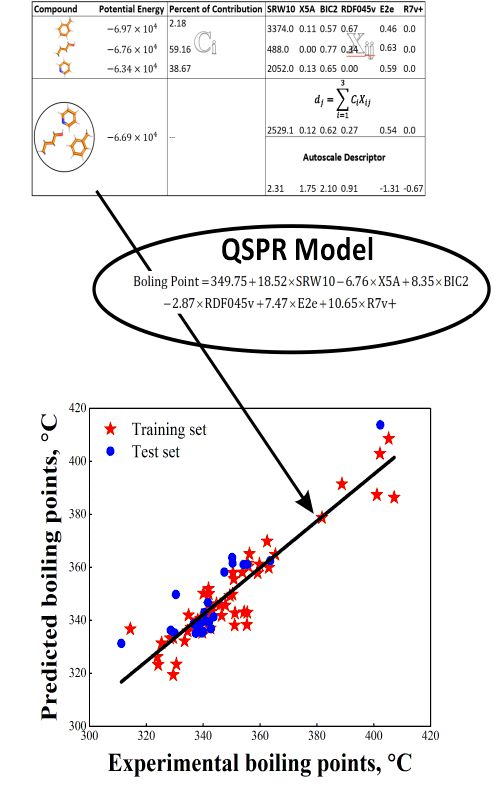
Recently, development of the QSPR models for mixtures has received much attention. The QSPR modelling of mixtures requires the use of the appropriate mixture descriptors. In this study, 12 mathematical equations were considered to compute mixture descriptors from the individual components for the prediction of normal boiling points of 78 ternary azeotropic mixtures. Multiple linear regression (MLR) was employed to build all QSPR models. Memorized_ACO algorithm was employed for subset variable selection. An ensemble model was also constructed using averaging strategy to improve the predictability of the final QSAR model. The models have been validated by a test set comprised of 24 ternary azeotropes and by different statistical tests. The resulted ensemble QSPR model had R2training, R2test and q2 of 0.97, 0.95, and 0.96, respectively. The mean absolute error (MAE), as a good indicator of model performance, were found to be 3.06 and 3.52 for training and testing sets, respectively.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
M. B. Franke, Comput. Chem. Eng. 89 (2016) 204 (https://doi.org/10.1016/j.compchemeng.2016.03.027)
Q.-K. Le, I. J. Halvorsen, O. Pajalic, S. Skogestad, Chem. Eng. Res. Des. 99 (2015) 111 (https://doi.org/10.1016/j.cherd.2015.03.022)
W. Li, L. Zhong, Y. He, J. Meng, F. Yao, Y. Guo, et al., Ind. Eng. Chem. Res. 54 (2015) 7668 (https://doi.org/10.1021/acs.iecr.5b00572)
Y. Wang, S. Liang, G. Bu, W. Liu, Z. Zhang, Z. Zhu, Ind. Eng. Chem. Res. 54 (2015) 12908 (https://doi.org/10.1021/acs.iecr.5b03666)
B. ZareNezhad, N. Hosseinpour, Energy Convers. Manage. 50 (2009) 1491 (https://doi.org/10.1016/j.enconman.2009.02.016)
Y. Tavan, S. Shahhosseini, Energy Technol. 4 (2016) 424 (https://doi.org/10.1002/ente.201500287)
K. Tochigi, D. Tiegs, J. Gmehling, K. Kojima, J. Chem. Eng. Jpn. 23 (1990) 453 (https://doi.org/10.1252/jcej.23.453)
S. M. Hosseini, M. M. Alavianmehr, D. Mohammad-Aghaie, F. Fadaei-Nobandegani, J. Moghadasi, J. Ind. Eng. Chem. 19 (2013) 769 (https://doi.org/10.1016/j.jiec.2012.10.013)
J. Gmehling, J. Li, M. Schiller, Ind. Eng. Chem. Res. 32 (1993) 178 (https://doi.org/10.1021/ie00013a024)
M. J. Hait, C. L. Liotta, C. A. Eckert, D. L. Bergmann, A. M. Karachewski, A. J. Dallas, et al., Ind. Eng. Chem. Res. 32 (1993) 2905 (https://doi.org/10.1021/ie00023a064)
S. Yousefinejad, F. Honarasa, H. Montaseri, RSC Adv. 5 (2015) 42266 (https://doi.org/10.1039/C5RA05930E)
A. Klamt, F. Eckert, Fluid Phase Equilib. 172 (2000) 43 (https://doi.org/10.1016/s0378-3812(00)00357-5)
D. E. Nanu, T. W. De Loos, Mol. Phys. 102 (2004) 235 (https://doi.org/10.1080/00268970410001655871)
A. A. Oliferenko, P. V. Oliferenko, J. S. Torrecilla, A. R. Katritzky, Ind. Eng. Chem. Res. 51 (2012) 9123 (https://doi.org/10.1021/ie202550v)
A. R. Katritzky, I. B. Stoyanova-Slavova, K. Tämm, T. Tamm, M. Karelson, J. Phys. Chem., A 115 (2011) 3475 (https://doi.org/10.1021/jp104287p)
I. Oprisiu, S. Novotarskyi, I. V. Tetko, J. Cheminform. 5 (2013) 4 (https://doi.org/10.1186/1758-2946-5-4)
V. Zare-Shahabadi, M. Lotfizadeh, A. R. A. Gandomani, M. M. Papari, J. Mol. Liq. 188 (2013) 222 (https://doi.org/10.1016/j.molliq.2013.09.037)
T. Gaudin, P. Rotureau, G. Fayet, Ind. Eng. Chem. Res. 54 (2015) 6596 (https://doi.org/10.1021/acs.iecr.5b01457)
Z. Faramarzi, F. Abbasitabar, V. Zare-Shahabadi, H. J. Jahromi, J. Mol. Liq. 296 (2019) 111854 (https://doi.org/10.1016/j.molliq.2019.111854)
Y. Demirel, Thermochim. Acta 339 (1999) 79 (https://doi.org/10.1016/s0040-6031(99)00211-7)
ChemDraw Ultra 6.0 and Chem3D Ultra, Cambridge Soft Corporation, Cambridge, MA
MOE, Chemical Computing Group Inc., Montreal (http://www.chemcomp.com)
R. Todeschini, V. Consonni, M. Pavan, Dragon Software Version 2.1, Chemometrics and QSAR Research Group, Milano, 2002
E. N. Muratov, E. V. Varlamova, A. G. Artemenko, P. G. Polishchuk, V. E. Kuz'min, Mol. Inform. 31 (2012) 202 (https://doi.org/10.1002/minf.201100129)
F. Abbasitabar, V. Zare-Shahabadi, SAR QSAR Environ. Res. 23 (2011) 1 (https://doi.org/10.1080/1062936x.2011.623316)
B. Hemmateenejad, M. Shamsipur, V. Zare-Shahabadi, M. Akhond, Anal. Chim. Acta 704 (2011) 57 (https://doi.org/10.1016/j.aca.2011.08.010)
V. Zare-Shahabadi, Med. Chem. Res. 25 (2016) 2787 (https://doi.org/10.1007/s00044-016-1666-z)
F. Abbasitabar, V. Zare-Shahabadi, Chemosphere 172 (2017) 249 (https://doi.org/10.1016/j.chemosphere.2016.12.095)
D. Baumann, K. Baumann, J. Cheminform. 6 (2014) 47 (https://doi.org/10.1186/s13321-014-0047-1)
D. L. Massart, B. G. M. Vandeginste, L. M. C. Buydens, S. De Jong, P. J. Lewi, J. Smeyers-Verbeke, Handbook of Chemometrics and Qualimetrics Part A, Elsevier, Amsterdam, 1997, pp. 286–288
S. Saaidpour, Phys. Chem. Res. 4 (2016) 61 (https://doi.org/10.22036/pcr.2016.11759)
F. Abbasitabar, V. Zare-Shahabadi, Drug Res (Stuttgart) 67 (2017) 476 (https://doi.org/10.1055/s-0043-108553)
K. Roy, S. Kar, P. Ambure, Chemom. Intell. Lab. Syst. 145 (2015) 22 (http://dx.doi.org/10.1016/j.chemolab.2015.04.013)
X. Bian, P. Diwu, Y. Liu, P. Liu, Q. Li, X. Tan, J. Chemom. 32 (2018) e2940 (https://doi.org/10.1002/cem.2940)
V. Zare-Shahabadi, F. Abbasitabar, M. Akhond, M. Shamsipur, J. Braz. Chem. Soc. 24 (2013) 1561 (http://dx.doi.org/10.5935/0103-5053.20130197)
S. Ma, S. Li, Ind. Eng. Chem. Res. 52 (2013) 543 (https://doi.org/10.1021/ie302909b)
A. A. Oliferenko, P. V. Oliferenko, J. S. Torrecilla, A. R. Katritzky, Ind. Eng. Chem. Res. 52 (2013) 545 (https://doi.org/10.1021/ie3033125)
S. Guariento, M. Tonelli, S. Espinoza, A. S. Gerasimov, R. R. Gainetdinov, E. Cichero, Eur. J. Med. Chem. 146 (2018) 171 (https://doi.org/10.1016/j.ejmech.2018.01.059)
O. Deeb, B. Hemmateenejad, Chem. Biol. Drug Des. 70 (2007) 19 (https://doi.org/10.1111/j.1747-0285.2007.00528.x)
R. Todeschini, V. Consonni, R. Mannhold, H. Kubinyi, G. Folkers. Molecular Descriptors for Chemoinformatics: Volume I: Alphabetical Listing / Volume II: Appendices, References, Wiley, New York, 2009, pp. 17–20.