In silico studies of phycobilins as potential candidates for inhibitors of viral proteins associated with COVID-19 Scientific paper
Main Article Content
Abstract
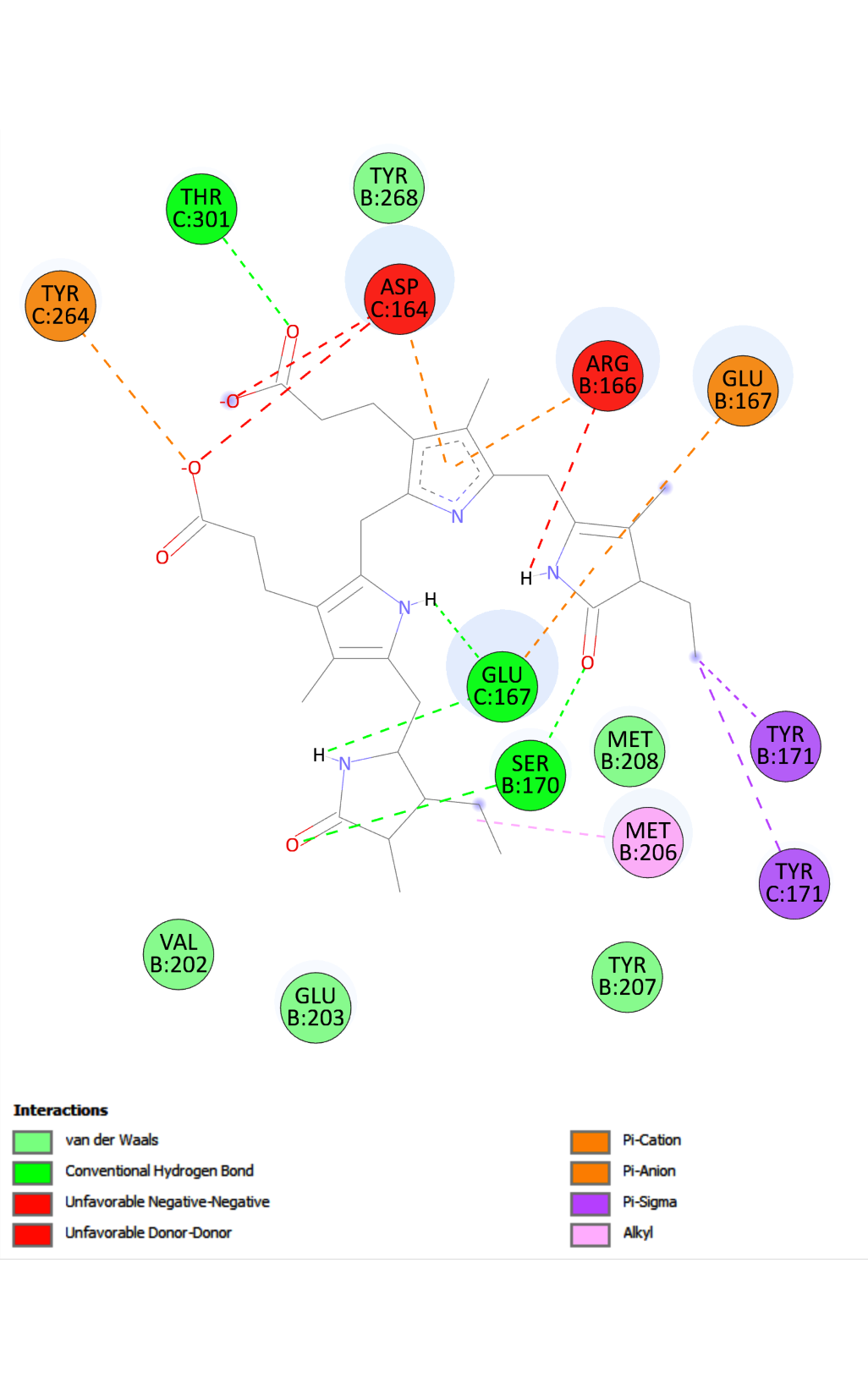
In this in silico study, it was investigated whether phycobilins (phycocyanobilin, phycoerythrobilin and phycourobilin) could be inhibitors of the activity of the main proteins of the SARS-CoV-2 virus. All chromophores exhibited a binding energy value of ≥–37 kJ mol-1 for PLpro-WT, PLpro-C111S, helicase-ANP binding site, Nsp3-macrodomain, Nsp3-MES site and Nsp10/14-N7-Mtase. Phycocyanobilin showed the highest binding energy of –44.77 kJ mol-1 against the target protein PLpro-C111S. It was found that, apart from the hydrogen bonds and hydrophobic interactions, phycobilins also form electrostatic interactions with the SARS-CoV-2 proteins. The network of non-covalent interactions was found to be important for the stability of the examined virus proteins. All phycobilins have good pharmacokinetic and drug-likeness properties. This study’s results suggest that the screened phycobilins could serve as promising drugs for the treatment of COVID-19 with further rigorous validation studies.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-66/2024-03/200026 and 451-03-66/2024-03/200168
References
A. E. Gorbalenya, S. C. Baker, R. S. Baric, R. J. de Groot, C. Drosten, A. A. Gulyaeva, B. L. Haagmans, C. Lauber, A. M. Leontovich, B. W. Neuman, D. Penzar, S. Perlman, L. L. M. Poon, D. V. Samborskiy, I. A. Sidorov, I. Sola, J. Ziebuhr, Nat. Microbiol. 5 (2020) 536 (https://doi.org/10.1038/s41564-020-0695-z)
S. Aboul-Fotouh, A. N. Mahmoud, E. M. Elnahas, M. Z. Habib, S. M. Abdelraouf, Virol. J. 20 (2023) 241 (https://doi.org/10.1186/s12985-023-02210-z)
A. von Delft, M. D. Hall, A. D. Kwong, L. A. Purcell, K. S. Saikatendu, U. Schmitz, J. A. Tallarico, A. A. Lee, Nat. Rev. Drug Discov. 22 (2023) 585 (https://doi.org/10.1038/s41573-023-00692-8)
T. F. Aiello, C. Garcia-Vidal, A. Soriano, Rev. Esp. Quimioter. 35 (2022) 10 (https://doi.org/10.37201/req/s03.03.2022)
C. C. Chang, H. J. Hsu, T. Y. Wu, J. W. Liou, Tzu Chi Med. J. 34 (2022) 276 (https://doi.org/10.4103/tcmj.tcmj_318_21)
D. Saxena, L. Batra, S. K. Verma, Pathogens 12 (2023) 823 (https://doi.org/10.3390/pathogens12060823)
M. S. Murgueitio, M. Bermudez, J. Mortier, G. Wolber, Drug Discov. Today Technol. 9 (2012) e219 (https://doi.org/10.1016/j.ddtec.2012.07.009)
P. Gale, Microb. Risk Anal. 21 (2022) 100198 (https://doi.org/10.1016/j.mran.2020.100140)
M. E. Popović, G. Šekularac, M. Popović, Microb. Risk Anal. 26 (2024) 100290 (https://doi.org/10.1016/j.mran.2024.100290)
M. Popović, M. Stevanović, M. Mihailović. J. Serb. Chem. Soc. 89 (2024) 305 (https://doi.org/10.2298/JSC240119019P)
C. H. Kim, Front. Pharmacol. 12 (2021) 590509 (https://doi.org/10.3389/fphar.2021.590509)
M. M. Rahman, M. R. Islam, S. Shohag, M. E. Hossain, M. Shah, S. K. Shuvo, H. Khan, M. A. R. Chowdhury, I. J. Bulbul, M. S. Hossain, S. Sultana, M. Ahmed, M. F. Akhtar, A. Saleem, M. H. Rahman, Environ. Sci. Pollut. Res. Int. 29 (2022) 46527 (https://doi.org/10.1007/s11356-022-20328-5)
R. Ghildiyal, V. Prakash, V. K. Chaudhary, V. Gupta, R. Gabrani, Phytochemicals as Antiviral Agents: Recent Updates. in M. K. Swamy (eds) Plant-derived Bioactives: Production, Properties and Therapeutic Applications. Singapore: Springer Singapore, 2020:279 (https://doi.org/10.1007/978-981-15-1761-7_12)
J. S. Mani, J. B. Johnson, J. C. Steel, D. A. Broszczak, P. M. Neilsen, K. B. Walsh, M. Naiker, Virus Res. 284 (2020) 197989 (https://doi.org/10.1016/j.virusres.2020.197989)
S. B. Kumar, S. Krishna, S. Pradeep, D. E. Mathews, R. Pattabiraman, M. Murahari, T. P. K. Murthy, Comput. Biol. Med. 134 (2021) 104524 (https://doi.org/10.1016/j.compbiomed.2021.104524)
R. C. Silva, H. F. Freitas, J. n. M. Campos, N. M. Kimani, C. H. T. P. Silva, R. S. Borges, S. S. R. Pita, C. B. R. Santos, Int. J. Mol. Sci. 22 (2021) 11739 (https://doi.org/10.3390/ijms222111739)
S. S. Gupta, A. Kumar, R. Shankar, U. Sharma, J. Mol. Graph. Model. 106 (2021) 107916 (https://doi.org/10.1016%2Fj.jmgm.2021.107916)
J. Li, K. T. McKay, J. M. Remington, S. T. Schneebeli, Sci. Rep. 11 (2021) 16307 (https://doi.org/10.1038/s41598-021-95826-6)
B. Pendyala, A. Patras, C. Dash, Front Microbiol. 12 (2021) 645713 (https://doi.org/10.3389/fmicb.2021.645713)
S. Geahchan, H. Ehrlich, M. A. Rahman, Marine Drugs 19 (2021) 409 (https://doi.org/10.3390/md19080409)
L. Tounsi, H. B. Hlima, F. Hentati, O. Hentati, H. Derbel, P. Michaud, S. Abdelkafi, Mar. Drugs 21 (2023) 440 (https://doi.org/10.3390/md21080440)
K. Liu, X. Lu, H. Shi, X. Xu, R. Kong, S. Chang, Nucleic Acids Res. 51 (2023) W365 (https://doi.org/10.1093/nar/gkad414)
O. Trott, A. J. Olson, J. Comput. Chem. 31 (2010) 455 (https://doi.org/10.1002/jcc.21334)
J. Eberhardt, D. Santos-Martins, A. F. Tillack, S. Forli, J. Chem. Inf. Model. 61 (2021) 3891 (https://doi.org/10.1021/acs.jcim.1c00203)
N. M. O'Boyle, M. Banck, C. A. James, C. Morley, T. Vandermeersch, G. R. Hutchison, J. Cheminformatics. 3 (2011) 33 (https://doi.org/10.1186/1758-2946-3-33)
S. Kim, J. Chen, T. Cheng, A. Gindulyte, J. He, S. He, Q. Li, B. A. Shoemaker, P. A. Thiessen, B. Yu, L. Zaslavsky, J. Zhang, E. E. Bolton, Nucleic Acids Res. 51 (2023) D1373-D1380 (https://doi.org/10.1093/nar/gkac956)
Biovia, D.S. (2021) Discovery Studio Visualizer. San Diego.
A. Daina, O. Michielin, V. Zoete, Sci. Rep. 7 (2017) 42717 (https://doi.org/10.1038/srep42717)
D. Shin, R. Mukherjee, D. Grewe, D. Bojkova, K. Baek, A. Bhattacharya, L. Schulz, M. Widera, A. R. Mehdipour, G. Tascher, P. P. Geurink, A. Wilhelm, G. van der Heden van Noort, H. Ovaa, S. Müller, K. P. Knobeloch, K. Rajalingam, B. A. Schulman, J. Cinatl, G. Hummer, S. Ciesek, I. Dikic, Nature 587 (2020) 657 (https://doi.org/10.1038/s41586-020-2601-5)
R. Ferreira de Freitas, M. Schapira, Med. Chem. Commun. 8 (2017) 1970 (https://doi.org/10.1039/C7MD00381A)
A. S. Mahadevi, G. N. Sastry, Chem. Rev. 116 (2016) 2775 (https://doi.org/10.1021/cr500344e)
R. Singh, N. Chauhan, M. Kuddus, Environ. Sci. Pollut. Res. Int. 28 (2021) 52798 (https://doi.org/10.1007/s11356-021-16104-6).