Optimizing alginate immobilization of food-derived C-phycocyanin: structural and functional characterization
Main Article Content
Abstract
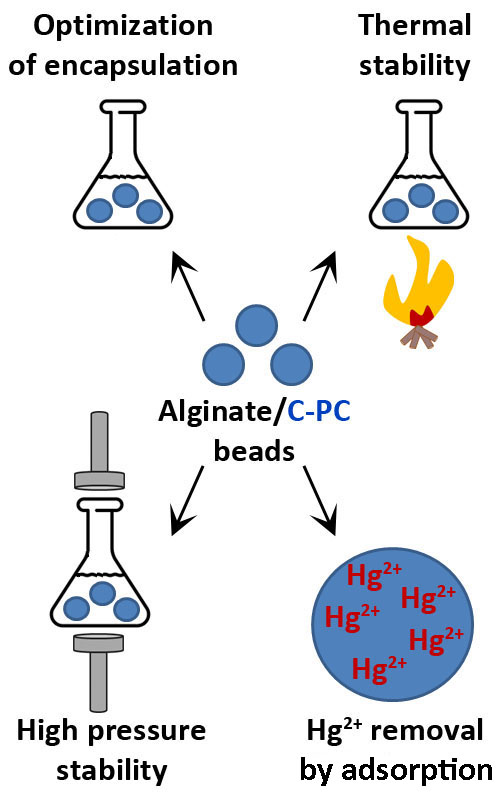
C-phycocyanin (C-PC) represents a significant component of the cyanobacteria Arthrospira platensis (Spirulina) biomass. Beyond its nutritional value, this protein exhibits numerous beneficial biological activities. A covalently attached chromophore, phycocyanobilin, gives C-PC a blue color, enabling its use as a natural food colorant. Additionally, phycocyanobilin exhibits various bioactive properties, including metal-binding activities. A key drawback to the broader industrial application of C-PC is its poor stability. Alternative food formulations using natural polymers as carriers and active components have recently gained considerable scientific attention. This paper describes optimized conditions for C-PC immobilization using alginate. The structural stabilization of immobilized C-PC was analyzed under high temperature (60°C) and high pressure (450 MPa). The storage stability of immobilized C-PC in dried alginate beads was tested by keeping the samples at 4°C for one month. The potential application of immobilized C-PC for the removal of mercury ions was also investigated. Alginate immobilization proved effective in stabilizing C-PC, significantly preserving its structure during prolonged storage, thermal treatment, and high-pressure exposure. Under the tested conditions, 97% of Hg2+ ions were removed by immobilized C-PC. Overall, this study optimized the procedure for enhancing C-PC stability through alginate immobilization and broadened its potential applications in food and bioremediation industries.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
L. Zhang, W. Liao, Y. Huang, Y. Wen, Y. Chu, C. Zhao, Food Prod. Process. Nutr. 4 (2022) 23 (https://doi.org/10.1186/s43014-022-00103-2)
V. Prete, A. C. Abate, P. Di Pietro, M. De Lucia, C. Vecchione, A. Carrizzo, Nutrients 16 (2024) 642 (https://doi.org/10.3390/nu16050642)
P. Grover, A. Bhatnagar, N. Kumari, A. Narayan Bhatt, D. Kumar Nishad, J. Purkayastha, Saudi J. Biol. Sci. 28 (2021) 1853 (https://doi.org/10.1016/j.sjbs.2020.12.037)
M. A. Domínguez-Martín, P. V. Sauer, H. Kirst, M. Sutter, D. Bína, B. J. Greber, E. Nogales, T. Polívka, C. A. Kerfeld, Nature 609 (2022) 835 (https://doi.org/10.1038/s41586-022-05156-4)
J. Dagnino-Leone, C. P. Figueroa, M. L. Castañeda, A. D. Youlton, A. Vallejos-Almirall, A. Agurto-Muñoz, J. Pavón Pérez, C. Agurto-Muñoz, Comput. Struct. Biotechnol. J. 20 (2022) 1506 (https://doi.org/10.1016/j.csbj.2022.02.016)
T. Minato, T. Teramoto, N. Adachi, N. K. Hung, K. Yamada, M. Kawasaki, M. Akutsu, T. Moriya, T. Senda, S. Ogo, Y. Kakuta, K.-S. Yoon, Commun. Biol. 4 (2021) 1238 (https://doi.org/10.1038/s42003-021-02767-x)
A. M. Saxena, J. Mol. Biol. 200 (1988) 579 (https://doi.org/10.1016/0022-2836(88)90544-X)
X.-Q. Wang, L.-N. Li, W.-R. Chang, J.-P. Zhang, L.-L. Gui, B.-J. Guo, D.-C. Liang, Acta Crystallogr. Sect. D Biol. Crystallogr. 57 (2001) 784 (https://doi.org/10.1107/S0907444901004528)
T. Watermann, H. Elgabarty, D. Sebastiani, Phys. Chem. Chem. Phys. 16 (2014) 6146 (https://doi.org/10.1039/C3CP54307B)
F. Pagels, A. C. Guedes, H. M. Amaro, A. Kijjoa, V. Vasconcelos, Biotechnol. Adv. 37 (2019) 422 (https://doi.org/10.1016/j.biotechadv.2019.02.010)
Y. Hou, M. Yan, Q. Wang, Y. Wang, Y. Xu, Y. Wang, H. Li, H. Wang, Food Anal. Methods 10 (2017) 1931 (https://doi.org/10.1007/s12161-016-0759-0)
M. Carocho, P. Morales, I. C. F. R. Ferreira, Trends Food Sci. Technol. 45 (2015) 284 (https://doi.org/10.1016/j.tifs.2015.06.007)
M. I. Landim Neves, E. K. Silva, M. A. A. Meireles, Trends Food Sci. Technol. 112 (2021) 163 (https://doi.org/10.1016/j.tifs.2021.03.023)
D. Pez Jaeschke, I. Rocha Teixeira, L. Damasceno Ferreira Marczak, G. Domeneghini Mercali, Food Res. Int. 143 (2021) 110314 (https://doi.org/10.1016/j.foodres.2021.110314)
R. Chaiklahan, N. Chirasuwan, B. Bunnag, Process Biochem. 47 (2012) 659 (https://doi.org/10.1016/j.procbio.2012.01.010)
S. Minić, N. Gligorijević, L. Veličković, M. Nikolić, Int. J. Mol. Sci. 25 (2024) 7187 (https://doi.org/10.3390/ijms25137187)
H. Wang, Z. Ouyang, L. Hu, Y. Cheng, J. Zhu, L. Ma, Y. Zhang, Food Chem. 397 (2022) 133725 (https://doi.org/10.1016/j.foodchem.2022.133725)
B.-W. Qiao, X.-T. Liu, C.-X. Wang, S. Song, C.-Q. Ai, Y.-H. Fu, Front. Nutr. 9 (2022) 890942 (https://doi.org/10.3389/fnut.2022.890942)
A. Londoño-Moreno, Z. Mundo-Franco, M. Franco-Colin, C. Buitrago-Arias, M. L. Arenas-Ocampo, A. R. Jiménez-Aparicio, E. Cano-Europa, B. H. Camacho-Díaz, Foods 12 (2023) 3272 (https://doi.org/10.3390/foods12173272)
M. Yan, B. Liu, X. Jiao, S. Qin, Food Bioprod. Process. 92 (2014) 89 (https://doi.org/10.1016/j.fbp.2013.07.008)
D. Rajmohan, D. Bellmer, Int. J. Food Sci. 2019 (2019) Article ID 7101279 (https://doi.org/10.1155/2019/7101279)
H. N. Pradeep, C. A. Nayak, J. Food Sci. Technol. 56 (2019) 4526 (https://doi.org/10.1007/s13197-019-03955-8)
W. Pan-utai, S. Iamtham, J. King Saud Univ. - Sci. 31 (2019) 1535 (https://doi.org/10.1016/j.jksus.2018.05.026)
Hadiyanto, M. Suzery, D. Setyawan, D. Majid, H. Sutanto, IOP Conf. Ser. Earth Environ. Sci. 55 (2017) 012030 (https://doi.org/10.1088/1755-1315/55/1/012030)
J. Radović, D. Popović, T. Ćurčić, L. Veličković, S. Lević, V. Pavlović, S. Minić, M. Nikolić, N. Gligorijević, Algal Res. 80 (2024) 103543 (https://doi.org/10.1016/j.algal.2024.103543)
M. R. Nikolic, S. Minic, M. Macvanin, D. Stanic-Vucinic, T. Cirkovic Velickovic, Analytical Protocols in Phycobiliproteins Analysis, in E. Jacob-Lopes, M. Isabel Queiroz, L. Queiroz Zepka (Eds.), Pigment. from Microalgae Handb., Springer Cham, Cham, Germany, 2020, pp. 179 (https://doi.org/10.1007/978-3-030-50971-2_8)
R. Lauceri, G. Chini Zittelli, G. Torzillo, Algal Res. 44 (2019) 101685 (https://doi.org/10.1016/j.algal.2019.101685)
G. Patil, K. S. M. S. Raghavarao, Biochem. Eng. J. 34 (2007) 156 (https://doi.org/10.1016/j.bej.2006.11.026)
H.-L. Wu, G.-H. Wang, W.-Z. Xiang, T. Li, H. He, Int. J. Food Prop. 19 (2016) 2349 (https://doi.org/10.1080/10942912.2015.1038564)
C. Chen, I. Liu, R. MacColl, D. S. Berns, Biopolymers 22 (1983) 1223 (https://doi.org/10.1002/bip.360220414)
M. Faieta, L. Neri, G. Sacchetti, A. Di Michele, P. Pittia, Food Res. Int. 132 (2020) 109093 (https://doi.org/10.1016/j.foodres.2020.109093)
G. Martelli, C. Folli, L. Visai, M. Daglia, D. Ferrari, Process Biochem. 49 (2014) 154 (https://doi.org/10.1016/j.procbio.2013.10.008)
L. Jespersen, L. D. Strømdahl, K. Olsen, L. H. Skibsted, Eur. Food Res. Technol. 220 (2005) 261 (https://doi.org/10.1007/s00217-004-1062-7)
H. Scheer, W. Kufer, Zeitschrift Für Naturforsch. C 32 (1977) 513 (https://doi.org/10.1515/znc-1977-7-806)
S. Minic, L. Velickovic, B. Annighöfer, A. Thureau, N. Gligorijevic, Z. Jovanovic, A. Brûlet, S. Combet, Protein Sci. 33 (2024) e5145 (https://doi.org/10.1002/pro.5145)
Y. Li, Z. Zhang, M. Paciulli, A. Abbaspourrad, J. Food Sci. 85 (2020) 727 (https://doi.org/10.1111/1750-3841.14842)
Z. Zhang, S. Cho, Y. Dadmohammadi, Y. Li, A. Abbaspourrad, Food Hydrocoll. 110 (2021) 106055 (https://doi.org/10.1016/j.foodhyd.2020.106055)
H. Shkolnikov Lozober, Z. Okun, G. Parvari, A. Shpigelman, Antioxidants 12 (2023) 568 (https://doi.org/10.3390/antiox12030568)
S. T. Moe, K. I. Draget, G. Skjåk-Bræk, O. Simdsrød, Carbohydr Polym. 19 (1992) 279 (https://doi.org/10.1016/0144-8617(92)90081-Z)
N. A. A. Qasem, R. H. Mohammed, D. U. Lawal, Npj Clean Water 4 (2021) 36 (https://doi.org/10.1038/s41545-021-00127-0).