Investigations on the role of cation–pi interactions in active centres of superoxide dismutase Scientific paper
Main Article Content
Abstract
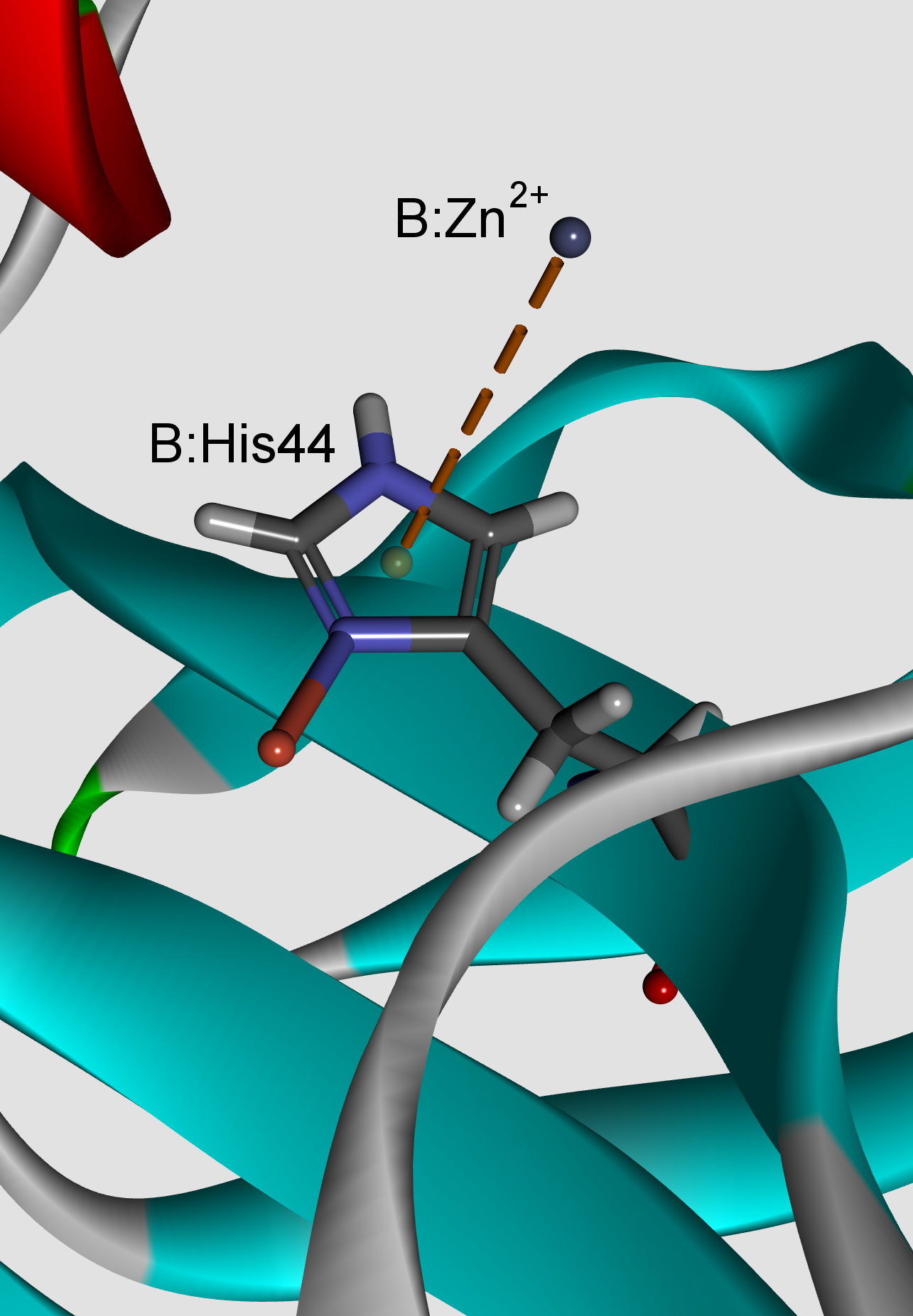
In this study, we have analysed the influence of cation–π interactions on stability and properties of superoxide dismutase (SOD) active centres. The number of interactions formed by arginine is higher than by lysine in the cationic group, while those formed by histidine are comparatively higher in the π group. The energy contribution resulting from most frequent cation–π interactions was in the lower range of strong hydrogen bonds. The cation–π interactions involving transition metal ions as cation have energy more negative than –418.4 kJ mol-1. The stabilization centres for these proteins showed that all the residues involved in cation–π interactions were important in locating one or more of such centres. The majority of the residues involved in cation–p interactions were evolutionarily conserved and might have a significant contribution towards the stability of SOD proteins. The results presented in this work can be very useful for understanding the contribution of cation–π interactions to the stability of SOD active centres.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
K. A. Dill, Biochemistry 29 (1990) 7133 (https://doi.org/10.1021/bi00483a001)
J. C. Ma, D. A. Dougherty, Chem. Rev. 97 (1997) 1303 (https://doi.org/10.1021/cr9603744)
K. S. Kim, P. Tarakeshwar, J. Y. Lee, Chem. Rev. 100 (2000) 4145 (https://doi.org/10.1021/cr990051i)
R. Wintjens, J. Liévin, M. Rooman, E. Buisine, J. Mol. Biol. 302 (2000) 393 (https://doi.org/10.1006/jmbi.2000.4040)
J. Cheng, W. Zhu, Y. Tang, Y. Xu, Z. Li, K. Chen, H. Jiang, Chem. Phys. Lett. 422 (2006) 455 (https://doi.org/10.1016/j.cplett.2006.03.005)
H. Ghiassi, H. Raissi, J. Sulfur Chem. 36 (2015) 48 (https://doi.org/10.1080/17415993.2014.962537)
N. Kumar, A. S. Gaur, G. N. Sastry, J. Chem. Sci. 133 (2021) 97 (https://doi.org/10.1007/s12039-021-01959-6)
S. M. Liao, Q. S. Du, J. Z. Meng, Z. W. Pang, R. B. Huang, Chem. Cent. J. 7 (2013) 44 (https://doi.org/10.1186/1752-153X-7-44)
S. Stojanović, Z. Petrović, M. Zlatović, J. Serb. Chem. Soc. 86 (2021) 781 (https://doi.org/10.2298/JSC210321042S)
S. Mecozzi, A. P. West, D. A. Dougherty, J. Am. Chem. Soc. 118 (1996) 2307 (https://doi.org/10.1021/ja9539608)
R. A. Kumpf, D. A. Dougherty, Science 261 (1993) 1708 (https://doi.org/10.1126/science.8378771)
D. Zhu, B. E. Herbert, M. A. Schlautman, E. R. Carraway, J. Environ. Qual. 33 (2004) 276 (https://doi.org/10.2134/jeq2004.2760)
L. M. Salonen, M. Ellermann, F. o. Diederich, Angew. Chem. Int. Ed. 50 (2011) 4808 (https://doi.org/10.1002/anie.201007560)
M. Moradi, A. A. Peyghan, Z. Bagheri, M. Kamfiroozi, J. Mol. Mod. 18 (2012) 3535 (https://doi.org/10.1007/s00894-012-1366-7)
U. D. Priyakumar, M. Punnagai, G. P. Krishna Mohan, G. N. Sastry, Tetrahedron 60 (2004) 3037 (https://doi.org/10.1016/j.tet.2004.01.086)
L. Brocchieri, S. Karlin, Proc. Natl. Acad. Sci. USA 91 (1994) 9297 https://doi.org/10.1073/pnas.91.20.9297)
P. W. Rose, B. Beran, C. Bi, W. F. Bluhm, D. Dimitropoulos, D. S. Goodsell, A. Prlic, M. Quesada, G. B. Quinn, J. D. Westbrook, J. Young, B. Yukich, C. Zardecki, H. M. Berman, P. E. Bourne, Nucleic Acids Res. 39 (2011) D392 (https://doi.org/10.1093/nar/gkq1021
J. M. Word, S. C. Lovell, J. S. Richardson, D. C. Richardson, J. Mol. Biol. 285 (1999) 1735 (https://doi.org/10.1006/jmbi.1998.2401)
Discovery Studio Visualizer, Release 2020, Accelrys Software Inc., Accelrys Software Inc., San Diego, CA, 2020
V. R. Ribić, S. Đ. Stojanović, M. V. Zlatović, Int. J. Biol. Macromol. 106 (2018) 559 (https://doi.org/10.1016/j.ijbiomac.2017.08.050)
J. Hostaš, D. Jakubec, R. A. Laskowski, R. Gnanasekaran, J. Řezáč, J. Vondrášek, P. Hobza, J. Chem. Theory Comput. 11 (2015) 4086 (http://dx.doi.org/10.1021/acs.jctc.5b00398)
Schrödinger Release 2018-1: Jaguar, Schrödinger, LLC, New York, 2018
T. H. Dunning, J. Chem. Phys. 90 (1989) 1007 (https://doi.org/10.1063/1.456153)
T. Clark, J. Chandrasekhar, G. n. W. Spitznagel, P. V. R. Schleyer, J. Comput. Chem. 4 (1983) 294 (https://doi.org/10.1002/jcc.540040303)
A. D. Bochevarov, E. Harder, T. F. Hughes, J. R. Greenwood, D. A. Braden, D. M. Philipp, D. Rinaldo, M. D. Halls, J. Zhang, R. A. Friesner, Int. J. Quantum Chem. 113 (2013) 2110 (https://doi.org/10.1002/qua.24481)
K. E. Riley, J. A. Platts, J. Řezáč, P. Hobza, J. G. Hill, J. Phys. Chem., A 116 (2012) 4159 (https://doi.org/10.1021/jp211997b)
G. J. Jones, A. Robertazzi, J. A. Platts, J. Phys. Chem., B 117 (2013) 3315 (https://doi.org/10.1021/jp400345s)
S. Saebø, W. Tong, P. Pulay, J. Chem. Phys. 98 (1993) 2170 (https://doi.org/10.1063/1.464195)
A. Reyes, L. Fomina, L. Rumsh, S. Fomine, Int. J. Quantum Chem. 104 (2005) 335 (https://doi.org/10.1002/qua.20558)
R. M. Balabin, J. Chem. Phys. 132 (2010) 231101 (https://doi.org/10.1063/1.3442466)
P. J. Hay, W. R. Wadt, J. Chem. Phys. 82 (1985) 299 (https://doi.org/10.1063/1.448975)
Y. Deng, B. t. Roux, J. Phys. Chem., B 113 (2009) 2234 (https://doi.org/10.1021/jp807701h)
J. C. Gumbart, B. t. Roux, C. Chipot, J. Chem. Theory Comput. 9 (2013) 794 (https://doi.org/10.1021/ct3008099)
Z. Dosztányi, A. Fiser, I. Simon, J. Mol. Biol. 272 (1997) 597 (https://doi.org/10.1006/jmbi.1997.1242)
Z. Dosztányi, C. Magyar, G. Tusnady, I. Simon, Bioinformatics 19 (2003) 899 (https://doi.org/10.1093/bioinformatics/btg110)
H. Ashkenazy, E. Erez, E. Martz, T. Pupko, N. Ben-Tal, Nucleic Acids Res. 38 (2010) W529 (https://doi.org/10.1093/nar/gkq399)
B. Boeckmann, A. Bairoch, R. Apweiler, M. C. Blatter, A. Estreicher, E. Gasteiger, M. J. Martin, K. Michoud, C. O'Donovan, I. Phan, S. Pilbout, M. Schneider, Nucleic Acids Res. 31 (2003) 365 (https://doi.org/10.1093/nar/gkg095)
A. S. Mahadevi, G. N. Sastry, Chem. Rev. 113 (2013) 2100 (https://doi.org/10.1021/cr300222d)
M. M. Gromiha, Biophys. Chem. 103 (2003) 251 (https://doi.org/10.1016/S0301-4622 (02)00318-6)
B. P. Dimitrijević, S. Z. Borozan, S. Đ. Stojanović, RSC Adv. 2 (2012) 12963 (https://doi.org/10.1039/C2RA21937A)
S. Z. Borozan, B. P. Dimitrijević, S. Đ. Stojanović, Comput. Biol. Chem. 47 (2013) 105 (https://doi.org/10.1016/j.compbiolchem.2013.08.005)
I. D. Mucić, M. R. Nikolić, S. Đ. Stojanović, Protoplasma 252 (2015) 947 (https://doi.org/10.1007/s00709-014-0727-8)
A. S. Mahadevi, G. N. Sastry, Chem. Rev. 116 (2016) 2775 (https://doi.org/10.1021/cr500344e)
D. Kim, E. C. Lee, K. S. Kim, P. Tarakeshwar, J. Phys. Chem., A 111 (2007) 7980 (https://doi.org/10.1021/jp073337x)
M. R. Davis, D. A. Dougherty, Phys. Chem. Chem. Phys. 17 (2015) 29262 (https://doi.org/10.1039/C5CP04668H)
G. R. Desiraju, T. Steiner, The Weak Hydrogen Bond, Oxford University Press, Oxford, 1999.