Influence of dissolved organic carbon nature on adsorption of ibuprofen, caffeine and diclofenac by powdered activated carbon from water Scientific paper
Main Article Content
Abstract
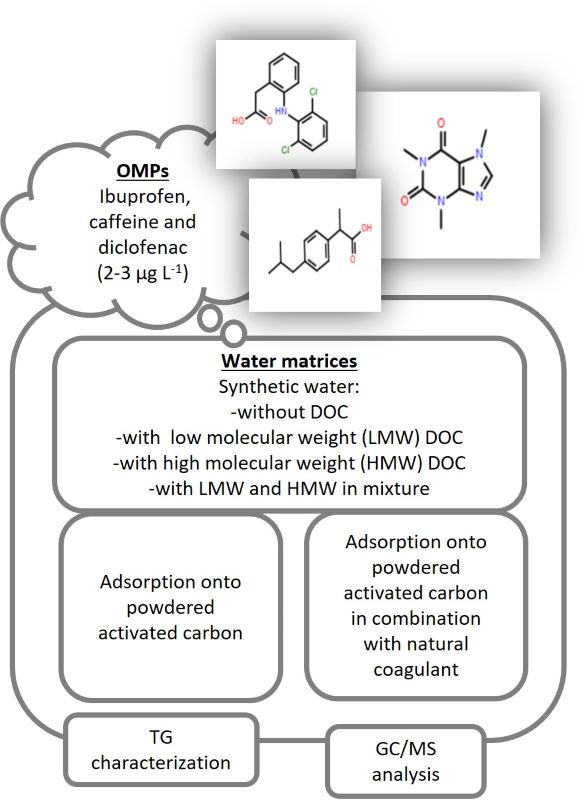
The impacts of structurally different dissolved organic carbon surrogates (DOC) on the adsorption of ibuprofen, caffeine and diclofenac (C0 2–3 µg L-1) onto powdered activated carbon (PAC) were studied. The efficiency of PAC in a synthetic matrix (SM) was tested when low or high molecular weight DOC surrogates were added. Additionally, the impact of a potential natural coagulant was investigated. Different DOC surrogates differently affected the adsorption of tested organic micropollutants (OMPs). In the first 30 min, a positive impact of all types of surrogates on the adsorption of neutral and hydrophilic caffeine was observed, while for negatively charged ibuprofen and diclofenac, the impacts varied. The low molecular weight DOC (containing amino acids and resorcinol) positively impacted the adsorption of ibuprofen, while effects were not clear for diclofenac. The high molecular weight DOC decreased the degree of adsorption for both compounds. The mass transfer calculations showed that low molecular weight DOC accelerated the transport of OMPs. High molecular weight DOC slowed reaching equilibrium (within 48 h). Thermogravimetry confirmed that the DOC coating qualitatively changes during the adsorption, which also affects the OMPs removal. The addition of a natural coagulant affected ibuprofen and diclofenac adsorption. For neutral caffeine the effects were not clear.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-66/2024-03/200125;451-03-65/2024-03/200125;451-03-137/2025-03/200125;451-03-136/2025-03/200125
References
T. Marjanović, M. Bogunović, S. Tenodi, V. Vasić, Đ. Kerkez, J. Prodanović, I. Ivančev-Tumbas, Sustainability 15 (2023) 9519 (https://doi.org/10.3390/su15129519)
I. Michael-Kordatou, C. Michael, X. Duan, X. He, D. D. Dionysiou, M. A. Mills, D. Fatta-Kassinos, Water Res. 77 (2015) 213 (https://doi.org/10.1016/j.watres.2015.03.011)
Q. L. Li, V. L. Snoeyink, C. Campos, B. J. Marinas, Environ. Sci. Technol. 36 (2002) 1510 (https://pubs.acs.org/doi/epdf/10.1021/es010870w)
Q. Li, V. L. Snoeyink, B. J. Marinas, C. Campos, Water Res. 37 (2003) 4863 (https://doi.org/10.1016/j.watres.2003.08.018)
P. Westerhoff, Y. Yoon, S. Snyder, E. Wert, Environ. Sci. Technol. 39 (2005) 6649 (https://doi.org/10.1021/es0484799)
D. J. de Ridder, A. R. D. Verliefde, S. G. J. Heijman, J. Q. J. C. Verberk, L. C. Rietveld, L. T. J. van der Aa, G. L. Amy, J. C. van Dijk, Water Sci. Technol. 63 (2011) 416 (https://doi.org/10.2166/wst.2011.237)
Y. Matsui, T. Yoshida, S. Nakao, D. R. U. Knappe, T. Matsushita, Water Res. 46 (2012) 4741 (http://dx.doi.org/10.1016/j.watres.2012.06.002)
F. Zietzschmann, E. Worch, J. Altmann, A. S. Ruhl, A. Sperlich, F. Meinel, M. Jekel, Water Res. 65 (2014) 297 (https://doi.org/10.1016/j.watres.2014.07.043)
R. Guillossou, J. Le Roux, S. Brosillon, R. Mailler, E. Vulliet, C. Morlay, F. Nauleau, V. Rocher, J. Gasperi, Chemosphere 245 (2020) 125530 (https://doi.org/10.1016/j.chemosphere.2019.125530)
R. Guillossou, J. Roux, R. Mailler, C. S. Pereira-Derome, G. Varrault, A. Bressy, E. Vulliet, C. Morlay, F. Nauleau, V. Rocher, J. Gasperi, Water Res. 172 (2020) 115487 (https://doi.org/10.1016/j.watres.2020.115487)
O. Autin, J. Hart, P. Jarvis, J. MacAdam, S. Parsons, B. Jefferson, Water Res. 47 (2013) 2041 (https://doi.org/10.1016/j.watres.2013.01.022)
C. Pelekani, V. Snoeyink, Carbon 38 (2000) 1423 (https://doi.org/10.1016/S0008-6223(99)00261-4)
C. Pelekani, V. Snoeyink, Carbon 39 (2001) 25 (https://doi.org/10.1016/S0008-6223(00)00078-6)
S. Dittmar, F. Zietzschmann, M. Mai, E. Worch, M. Jekel, A. S. Ruhl, Environ. Sci. Technol. 52 (2018) 7859 (https://doi.org/10.1021/acs.est.8b01503)
K. K. Shimabuku, J. M. Paige, M. Luna-Aguero, R. S. Summers, Environ. Sci. Technol. 51 (2017) 10031 (https://doi.org/10.1021/acs.est.7b00758)
J. J. Zhang, J. Zhai, H. Zheng, X. Li, Y. Wang, X. Li, B. Xing, Sci. Total Environ. 738 (2020) 139685 (https://doi.org/10.1016/j.scitotenv.2020.139685)
Q. Wang, R. L. Mitchell, R. Hofman, J. Yu, M. Yang, L. C. Rietveld, F. Zietzschmann, Water Res. 202 (2021) 117443 (https://doi.org/10.1016/j.watres.2021.117443)
Q. Wang, O. J. Lechtenfeld, L. C. Rietveld, J. Schuster, M. Ernst, R. Hofman-Caris, J. Kaesler, C. Wang, M. Yang, J. Yu, F. Zietzschmann, Environ. Sci. Ecotechnol. 21 (2024) 100392 (https://doi.org/10.1016/j.ese.2024.100392)
M. Bogunović, I. Ivančev-Tumbas, M. Česen, T. Đaković Sekulić, J. Prodanović, A. Tubić, D. Heath, E. Heath, Sci. Total Environ. 765 (2021) 142764 (https://doi.org/10.1016/j.scitotenv.2020.142764)
I. Ivančev-Tumbas, M. Bogunović, V. Vasić, M. Šćiban, A. Tubić, A. Leovac Maćerak, J. Prodanović, Environ. Technol. 43 (2022) 1163 (https://doi.org/10.1080/09593330.2020.1821788)
L. Kovalova, D. R. U. Knappe, K. Lehnberg, C. Kazner, J. Hollender, Environ. Sci. Pollut. Res. 20 (2013) 3607 (https://doi.org/10.1007/s11356-012-1432-9)
ChemSpider properties predicted by ACD/Labs, Online, https://www.chemspider.com/ (accessed on February 19, 2024)
E. R. V. Dickenson, J. E. Drewes, Water Sci. Technol. 62 (2010) 2270 (https://doi.org/10.2166/wst.2010.497)
J. Prodanović, PhD Thesis, University of Novi Sad, 2015
Q. Wang, F. Zietzschmann, R. Hofman-Caris, N. Jiang, J. Schuster, Z. Wang, J. Yu, M. Yang, L. C. Rietveld, Sep. Purif. Technol. 292 (2022) 120942 (https://doi.org/10.1016/j.seppur.2022.120942).