Electrocatalytic hydrogen evolution upon reduction of pyridoxal semicarbazone and thiosemicarbazone-based Cu(II) complexes Scientific paper
Main Article Content
Abstract
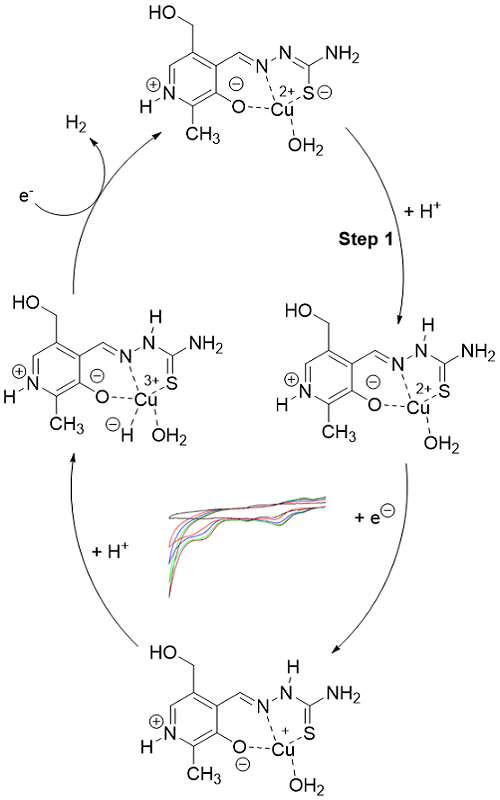
The growing global demand for renewable energy sources has pushed renewable, green energy sources to the forefront, among which the production of hydrogen gas from water occupies a significant place. To realize this goal, researchers across the globe are developing various systems that could swiftly catalyze the hydrogen evolution reaction (HER) in the highest possible yield. In the present work, the electrocatalytic HER performances of pyridoxal semicarbazone- and thiosemicarbazone-based Cu(II) complexes, i.e., ([Cu(PLSC)Cl2] and [Cu(PLTSC-H)H2O]Br·H2O) are reported. It has been unambiguously demonstrated that the complexes exhibit enviable level of HER catalytic activity. The catalytic activity of the complexes was not only the function of central metal but it was also controlled by the nature of the coordinating ligand.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
References
J. Zhu, L. Hu, P. Zhao, L. Lee, K. Wong, Chem. Rev. 120 (2020) 851 (https://doi.org/10.1021/acs.chemrev.9b00248)
J. Yu, Y. Dai, Q He, D. Zhao, Z. Shao, M. Ni, MRE 120 (2021)100024 (https://doi.org/10.1016/j.matre.2021.100024
Y. She, Z. Lyu, M. Zhao, R. Chen, Q. Nguyen, Y. Xia, Chem. Rev. 121 (2021) 649 (https://doi.org/10.1021/acs.chemrev.0c00454)
Z. Zhou, Z. Pei, L. Wei, S. Zhao, X. Jian,Y. Chen, Energy Environ. Sci. 13 (2020) 3185 (https://doi.org/10.1039/D0EE01856B)
S. Roy, Z. Huang, A. Buhunia, A. Castner, A. Gupta, X. Zou, S. Ott, J. Am. Chem. Soc. 141 (2019) 15942 (https://doi.org/10.1021/jacs.9b07084)
C. Chen, T. Chiou, H. Chang, W. Li, C. Tung, W. Liaw, Sustain. Energy Fuels 3 (2019) 2205 (https://doi.org/10.1039/C9SE00371A)
N. Cheng, S. Stambula, D. Wang, M. Banis, J. Liu, A. Riese, B. Xiao, R. Li, T. Sham, L. Liu, G. Botton, X. Sun, Nat. Commun. 7 (2016) 13638 (https://doi.org/10.1038/ncomms13638)
H. Tang, E. N. Brothers, C. A. Grapperhaus, M. B. Hall, ACS Catal. 10 (2020) 3778 (https://doi.org/10.1021/acscatal.9b04579)
H. Shao, S. K. Muduli, P. D. Tran, H. S. Soo, Chem. Commun. 52 (2016) 2948 (https://doi.org/10.1039/C5CC09456A)
A. Z. Haddad, B. D. Garabato, P. M. Kozlowski, R. M. Buchanan, C. A. Grapperhaus, J. Am. Chem. Soc. 138, 25 (2016) 7844 (https://doi.org/10.1021/jacs.6b04441)
V. Jevtovic, Cu, Fe, Ni and V Complexes with Pyridoxal Semicarbazones, Lap Lambert Publication, Saarbrücken, 2010 (https://www.amazon.com/complexes-pyridoxal-semicarbazones-properties-structural/dp/3838351339)
V. M. Leovac, V. S. Jevtovic, L. S. Jovanovic,G. A. Bogdanovic, J. Serb. Chem. Soc. 70 (2005) 423 (https://doi.org/10.2298/JSC0503393L)
S.AShidhani, M. Al Bouromi, S. Al Ameri, S. Al Ghawi, V. Jevtovic, Am. J. Chem. 6 (2016) 8 (https://doi.org/10.5923/j.chemistry.20160601.02)
V. Jevtovic, D. Vidovic, Acta Cryst., E 66(Pt.4) (2010) 408 (https://doi.org/10.1107/S1600536810003570)
V. Jevtovic, D. Cvetkovic, D. Vidovic, JICS 8 (2011)727 (https://doi.org/10.1007/BF03245904)
D. Vidovic, A. Radulovic,V. Jevtovic, Polyhedron 30 (2011) 16 (https://doi.org/10.1016/j.poly.2010.09.022)
N. Knezevic,V. Leovac,V. Jevtovic, S. Grguric-Sipka, T. Sabo, Inorg. Chem. Comm. 6 (2003) 561 (https://doi.org/10.1016/S1387-7003(03)00041-8)
R. Manikandan, P. Anitha, G. Prakash, P. Vijayan, P. Viswanathamurthi, Polyhedron 81 (2014) 619 (https://doi.org/10.1016/j.poly.2014.07.018)
R. Manikandan, P. Anitha, P. Viswanathamurthi, J. G. Maleck, Polyhedron 119 (2016) 300 (https://doi.org/10.1016/j.poly.2016.09.005)
R. Manikandana, P. Anithaa, G. Prakasha, P. Vijayana, P. Viswanathamurthi, R. Jay Butcher,J. G. Malecki, J. Mol. Catal., A 398 (2015) 312 (https://doi.org/10.1016/j.poly.2016.09.005)
J. Pisk, B. Prugovecki, D. Matkovic-Calogović, R. Poli, D. Agustin ,V. Vrdoljak, Polyhedron 33 (2012) 441 (https://doi.org/10.1016/j.poly.2011.12.003)
D. Perrin, W. Armarego, D. Perrin, Purification of Laboratory Chemicals, Pergamon, New York, 1988 (https://doi.org/10.1002/recl.19881071209)
V. Jevtovic, K. Alenezi, H. El Moll, A. Haque, J. Humaidi, S. A. Al-Zahrani, D. Vidovic, Int. J. Electrochem. Sci. 16 (2021) 210731 (https://doi.org/10.20964/2021.07.61)
T. Liu, D. L. Du Bois, R. M. Bullock, Nat. Chem. 5 (2013) 228 (https://doi.org/10.1038/nchem.1571)
B. H. Solis, S. Hammes-Schiffer, Inorg. Chem. 53 (2014) 6427 https://doi.org/10.1021/ic5002896
M. Drosou, F. Kamatsos, C. A. Mitsopoulou, Inorg. Chem. Front. 7 (2020) 37 (https://doi.org/10.1039/C9QI01113G).