Synthesis and biological activity of alkylthio and arylthio derivatives of tert-butylquinone Scientific paper
Main Article Content
Abstract
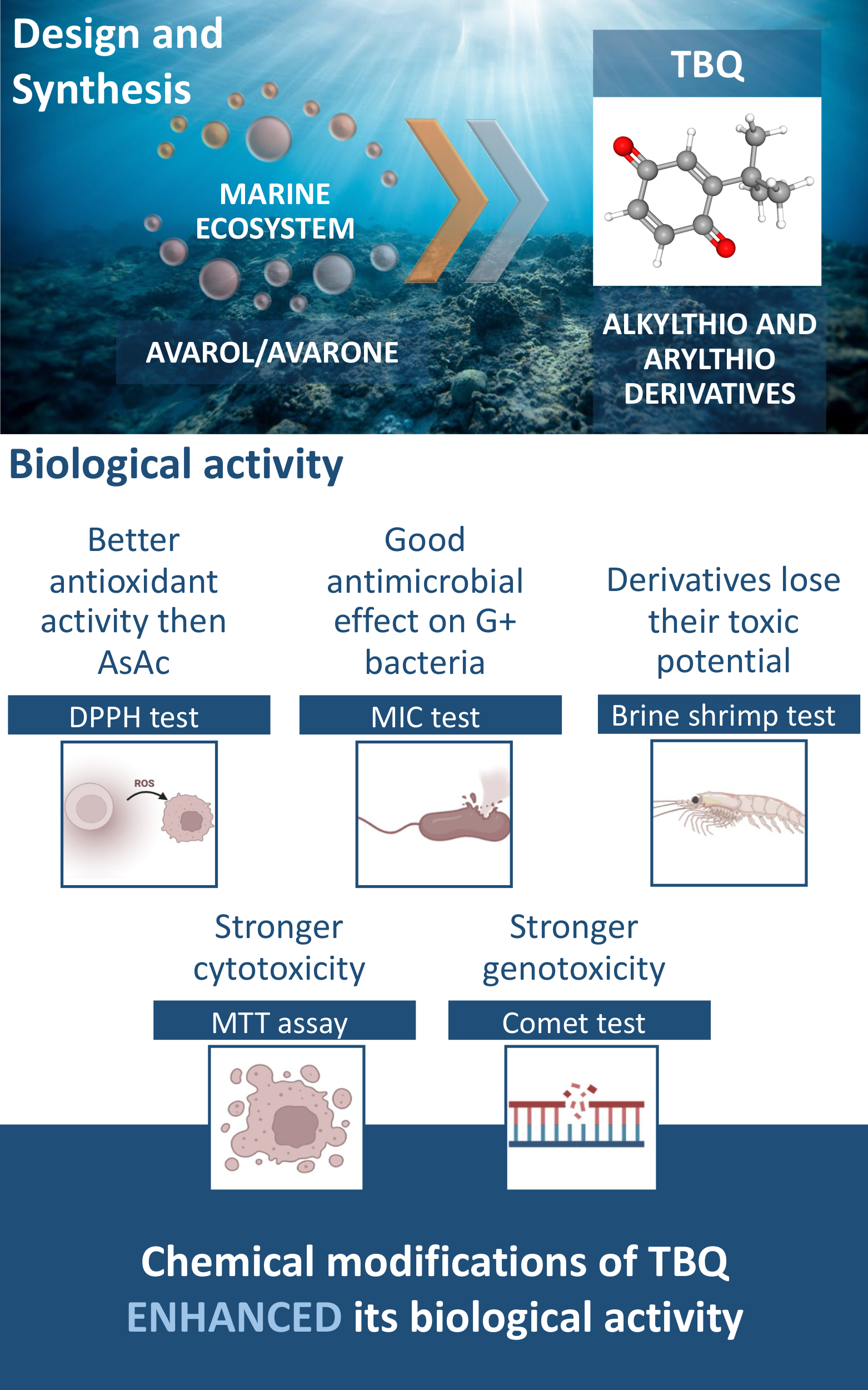
Biological activity of 2-tert-butyl-1,4-benzoquinone (TBQ) and its derivatives, 2-tert-butyl-5-(2-propylthio)-1,4-benzoquinone, 2-tert-butyl-5-(propylthio)-1,4-benzoquinone, 2-tert-butyl-5,6-(ethylenedithio)-1,4-benzoquinone, 2-tert-butyl-5-(phenylthio)-1,4-benzoquinone and 2-tert-butyl-6-(phenylthio)-1,4-benzoquinone, were tested for their antioxidant, antibacterial, toxic, cytotoxic and genotoxic potential. Using the DPPH test, all derivatives showed good antioxidant activity, better than ascorbic acid, and the 2-tert-butyl-5-(propylthio)-1,4-benzoquinone derivative showed the strongest effect. Better antibacterial potential was observed against Gram-positive bacteria in the broth microdilution method in which the 2-tert-butyl-5-(phenylthio)-1,4-benzoquinone derivative showed the strongest activity (MIC = 15.6 µM). The results of toxicity tests, using the Brine shrimp test, indicated that the derivatives lose their toxic potential compared to TBQ, except for 2-tert-butyl-6-(phenylthio)-1,4-benzoquinone, which showed a 3 times stronger effect. Cytotoxicity was assessed by the MTT assay in 24 and 72 h treatments in MRC-5, HS 294T and A549 cell lines in threefold decreasing gradient (11, 33 and 100 μM). Modifications potentiate the cytotoxic effect, and the strongest effect was observed with the 2-tert-butyl-5,6-(ethylendithio)-1,4-benzoquinone derivative. In addition, the genotoxic potential was examined in the MRC-5 cell line using the comet assay. All tested derivatives of TBQ showed a genotoxic effect at all applied subtoxic concentrations. In general, the chemical modifications of TBQ enhanced its biological activity.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-9/2021-14/200053;451-03-9/2021-14/200007;451-03-68/2022-14/200178;451-03-9/2021-14/200168;451-03-68/2022-14/200026
References
A. Upadhyay, Genes Dis. 8 (2021) 655 (https://doi.org/10.1016/j.gendis.2020.09.002)
IARC, Press Release 263 (2018) (https://www.iarc.who.int/wp-content/uploads/2018/09/pr263_E.pdf) (accessed on March 4, 2022)
J. Khazir, B. A. Mir, S. A. Mir, D. Cowan, J. Asian Nat. Prod. Res. 15 (2013) 764 (https://doi.org/10.1080/10286020.2013.798314)
R. G. Amaral, S. A. dos Santos, L. N. Andrade, P. Severino, A. A. Carvalho, Clin. Oncol 4 (2019) 1562 (https://www.clinicsinoncology.com/open-access/natural-products-as-treatment-against-cancer-a-historical-and-current-vision-1716.pdf)
J. T. Jimenez, M. Sturdíkova, E. Sturdík, Acta Chim. Slov. 2 (2009) 63 (http://acs.chtf.stuba.sk/papers/acs_0047.pdf)
E. Batke, R. Ogura, P. Vaupel, K. Hummel, F. Kallinowski, M. J. Gasić, H. C. Schröder, W. E. G. Müllerm, Cell Biochem. Funct. 6 (1988) 123 (https://doi.org/10.1002/cbf.290060207)
M. L. Ferrándiz, M. J. Sanz, G. Bustos, M. Payá, M. J. Alcaraz, S. de Rosa, Eur. J. Pharmacol. 253 (1994) 75 (https://doi.org/10.1016/0014-2999(94)90759-5)
M. Tsoukatou, J. P. Maréchal, C. Hellio, I. Novaković, S. Tufegdzic, D. Sladić, M. J. Gašić, A. S. Clare, C. Vagias, V. Roussis, Molecules 12 (2007) 1022 (https://doi.org/10.3390/12051022)
N. Aktaş, B. Gözcelioğlu, Y. Zang, W.-H. Lin, B. Konuklugil, FABAD J. Pharm. Sci. 35 (2010) 119 (http://dergi.fabad.org.tr/pdf/volum35/issue3/119-123.pdf)
T. Božić, I. Novaković, M. J. Gašić, Z. Juranić, T. Stanojković, S. Tufegdžić, Z. Kljajić, D. Sladić, Eur. J. Med. Chem. 45 (2010) 923 (https://doi.org/10.1016/j.ejmech.2009.11.033)
S. Kolarević, D. Milovanović, M. Kračun-Kolarević, J. Kostić, K. Sunjog, R. Martinović, J. Đorđević, I. Novaković, D. Sladić, B. Vuković-Gačić, Drug Chem. Toxicol. 42 (2019) 130 (https://doi.org/10.1080/01480545.2017.1413108)
M. Jeremić, J. Dinić, M. Pešić, M. Stepanovic, I. Novaković, D. Šegan, D. Sladić, J. Serb. Chem. Soc. 83 (2018) 1193 (https://doi.org/10.2298/JSC180627062J)
J. Vilipić, I. Novaković, T. Stanojković, I. Matić, D. Šegan, Z. Kljajić, D. Sladić, Bioorg. Med. Chem. 23 (2015) 6930 (https://doi.org/10.1016/j.bmc.2015.09.044)
M. Jeremić, M. Pešić, J. Dinić, J. Banković, I. Novaković, D. Šegan, D. Sladić, Eur. J. Med. Chem. 118 (2016) 107 (https://doi.org/10.1016/j.ejmech.2016.04.011)
J. Đorđević, S. Kolarević, J. Jovanović, J. Kostić-Vuković, I. Novaković, M. Jeremić, D. Sladić, B. Vuković-Gačić, Drug Chem. Toxicol. 43 (2020) 522 (https://doi.org/10.1080/01480545.2018.1514043)
X. Li, J. Ni, Y. Tang, X. Wang, H. Tang, H. Li, S. Zhang, X. Shen, Nat. Prod. Res. 33 (2019) 2722 (https://doi.org/10.1080/14786419.2018.1465425)
M. Sarvizadeh, O. Hasanpour, Z. Naderi Ghale-Noie, S. Mollazadeh, M. Rezaei, H. Pourghadamyari, M. Masoud Khooy, M. Aschner, H. Khan, N. Rezaei, L. Shojaie, H. Mirzaei, Front. Oncol. 11 (2021) 650256 (https://doi.org/10.3389/fonc.2021.650256)
M. C. H. Gruhlke, C. Nicco, F. Batteux, A. J. Slusarenko, Antioxidants 6 (2017) 1 (https://doi.org/10.3390/antiox6010001)
I. Novaković, Z. Vujčić, T. T. Božić, N. Božić, N. B. Milosavić, D. Sladić, J. Serb. Chem. Soc. 68 (2003) 243 (https://doi.org/10.2298/JSC0305243N)
M. S. Blois, Nature 181 (1958) 1199 (http://dx.doi.org/10.1038/1811199a0)
S. D. Sarker, L. Nahar, Y. Kumarasamy, Methods 42 (2007) 321 (https://doi.org/10.1016/j.ymeth.2007.01.006)
E. J. Crevelin, S. C. Caixeta, H. J. Dias, M. Groppo, W. R. Cunha, C. H. G. Martins, A. E. M. Crotti, Evid. Based Complementary Altern. Med. (2015) 102317 (https://doi.org/10.1155/2015/102317)
P. Vanhaecke, G. Persoone, Ecotoxicol. Test. Mar. Environ. 2 (1984) 588 (https://www.researchgate.net/publication/36455047)
A. Azqueta, K. B. Gutzkow, C. C. Priestley, S. Meier, J. S. Walker, G. Brunborg, A. R. Collins, Toxicol. in Vitro 27 (2013) 768 (https://doi.org/10.1016/j.tiv.2012.12.006)
Z. Gačić, S. Kolarević, K. Sunjog, M. Kračun-Kolarević, M. Paunović, J. Knežević-
-Vukčević, B. Vuković-Gačić, Environ. Pollut. 191 (2014) 145 (https://doi.org/10.1016/j.envpol.2014.04.024)
H. Lai, Y. Lim, Int. J. Environ. Sci. 2 (2011) 442 (https://doi.org/10.7763/IJESD.2011.V2.166)
S. A. Soto-Rodriguez, A. Roque, M. L. Lizarraga-Partida, A. L. Guerra-Flores, B. Gomez-Gil, Dis. Aquat. Org. 53 (2003) 231 (https://doi.org/10.3354/dao053231)
A. Koch, P. Tamez, J. Pezzuto, D. Soejarto, J. Ethnopharmacol. 101 (2005) 95 (https://doi.org/10.1016/j.jep.2005.03.011)
P. Tanamatayarat, P. Limtrakul, S. Chunsakaow, C. Duangrat, Thai J. Pharm. Sci. 27 (2003) 167 (https://www.thaiscience.info/Article%20for%20ThaiScience/Article/5/10016408.pdf)
D. Sladic, M. J. Gasic, Molecules 11 (2006) 1 (https://doi.org/10.3390/11010001)
I. Pérez-Torres, V. Guarner-Lans, M. E. Rubio-Ruiz, Int. J. Mol. Sci. 18 (2017) 2098 (https://doi.org/10.3390/ijms18102098)
C. P. Wu, S. Ohnuma, S. V. Ambudkar, Curr. Pharm. Biotechnol. 12 (2011) 609 (https://doi.org/10.2174/138920111795163887)
T. Okubo, Y. Yokoyama, K. Kano, I. Kano, Food Chem. Toxicol. 41 (2003) 679 (https://doi.org/10.1016/S0278-6915(03)00002-4)
W. Fiers, R. Beyaert, W. Declercq, P. Vandenabeele, Oncogene 18 (1999) 7719 (https://doi.org/10.1038/sj.onc.1203249).