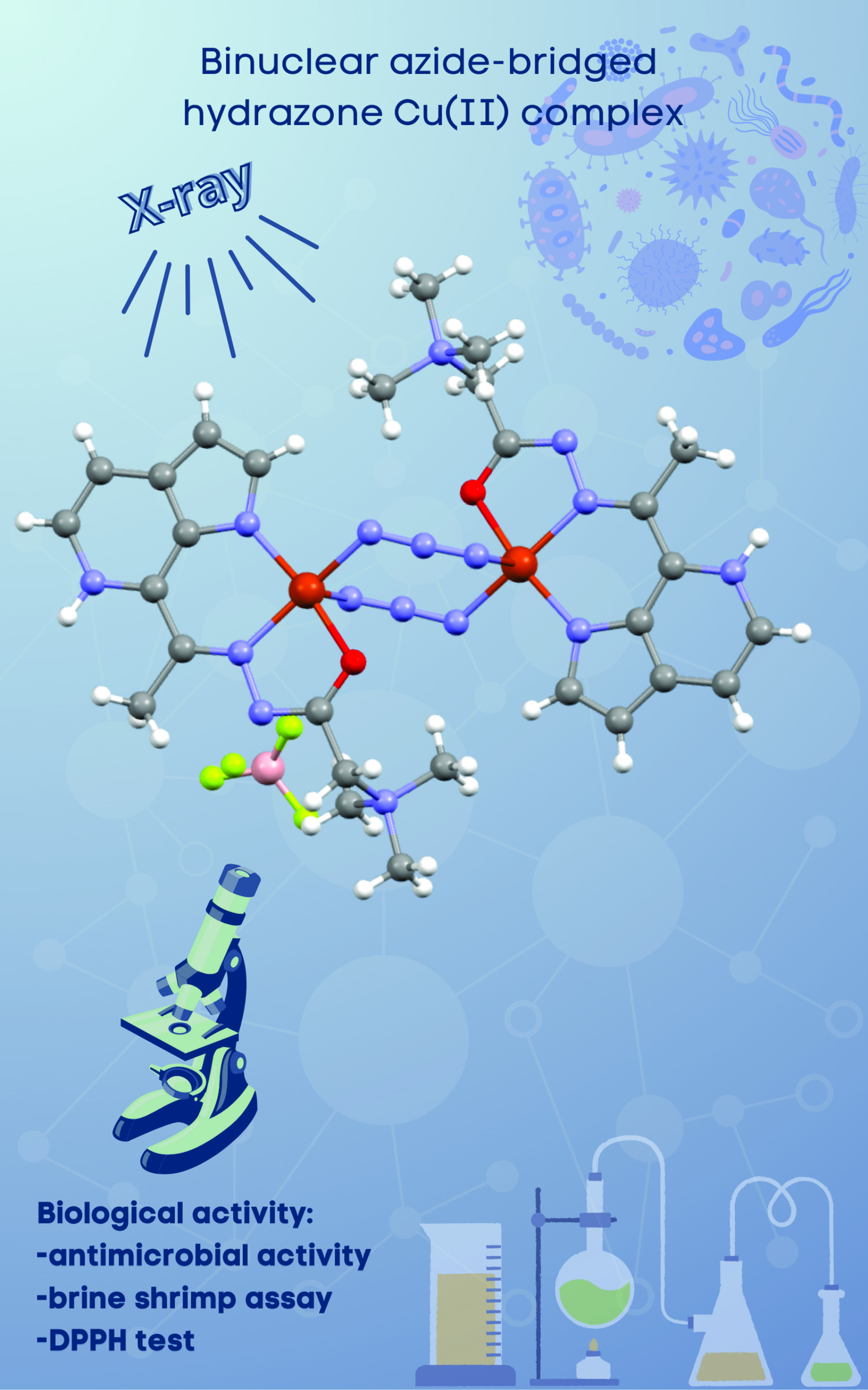
Binuclear azide-bridged hydrazone Cu(II) complex: Synthesis, characterization and evaluation of biological activity Scientific paper
Main Article Content
Abstract
The condensation product of 7-acetyl-6-azaindole and Girard’s T reagent ((E)-2-(2-(1-(1H-pyrrolo[2,3-c]pyridin-7-yl)ethylidene)hydrazineyl)-N,N,N-trimethyl-2-oxoethan-1-aminium, HL ligand) was used as a ligand in the reaction with Cu(BF4)2·6H2O and NaN3. The reaction led to the formation of a binuclear Cu(II) complex containing two end-to-end (di-m-1,3-N3) azide bridges, as well as two NNO-donor hydrazone ligands, forming an axially elongated square pyramidal geometry around each Cu(II) center. This end-to-end (di-m-1,3-N3) azide bridge binding mode has not yet been reported, in Cu(II) complexes containing the NNO-donor hydrazone ligands, which makes the structure of the complex even more interesting for further studies. The complex was characterized by elemental analysis, IR spectroscopy and X-ray crystallography, and it was found that it crystallizes in the triclinic space group P–1 with the asymmetric unit comprising one Cu(II) centre, zwitterionic ligand L, one azide (N3-) ligand and BF4- counter anion. Examination of antimicrobial activity of the complex shows higher antifungal and antibacterial activity towards tested Gram-positive bacteria in comparison to the hydrazone ligand, with the antifungal activity of the complex even being comparable to the activity of amphotericin B.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja,Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-47/2023-01/200026;451-03-47/2023-01/200168;451-03-47/2023-01/200288 -
Science Fund of the Republic of Serbia
Grant numbers 7750288 -
Javna Agencija za Raziskovalno Dejavnost RS
Grant numbers P1-0175
References
C. Adhikary, S. Koner, Coord. Chem. Rev. 254 (2010) 2933 (https://doi.org/10.1016/j.ccr.2010.06.001)
A. Escuer, G. Aromí, Eur. J. Inorg. Chem. 2006 (2006) 4721 (https://doi.org/10.1002/ejic.200600552)
K. Dankhoff, B. Weber, CrystEngComm 20 (2018) 818 (https://doi.org/10.1039/C7CE02007D)
M. R. Milenković, B. Čobeljić, K. Anđelković, I. Turel, Eur. J. Inorg. Chem. 2018 (2018) 838 (https://doi.org/10.1002/ejic.201701387)
P. Bhowmik, A. Bhattacharyya, K. Harms, S. Sproules, S. Chattopadhyay, Polyhedron 85 (2015) 221 (https://doi.org/10.1016/j.poly.2014.08.021)
M. Das, B. K. Shaw, B. N. Ghosh, K. Rissanen, S. K. Saha, S. Chattopadhyay, J. Coord. Chem. 68 (2015) 1361 (https://doi.org/10.1080/00958972.2015.1014350)
P. Mukherjee, O. Sengupta, M. G. B. Drew, A. Ghosh, Inorganica Chim. Acta 362 (2009) 3285 (https://doi.org/10.1016/j.ica.2009.02.041)
M. S. Ray, A. Ghosh, R. Bhattacharya, G. Mukhopadhyay, M. G. B. Drew, J. Ribas, Dalton Trans. (2004) 252 (https://doi.org/10.1039/B311499F)
S. Sen, S. Mitra, D. L. Hughes, G. Rosair, C. Desplanches, Polyhedron 26 (2007) 1740 (https://doi.org/10.1016/j.poly.2006.12.015)
M. M. Fousiamol, M. Sithambaresan, K. K. Damodaran, M. R. P. Kurup, Inorg. Chim. Acta 501 (2020) 119301 (https://doi.org/10.1016/j.ica.2019.119301)
T. Vitomirov, F. Dimiza, I. Z. Matić, T. Stanojković, A. Pirković, L. Živković, B. Spremo-Potparević, I. Novaković, K. Anđelković, M. Milčić, G. Psomas, M. Š. Ristović, J. Inorg. Biochem. 235 (2022) 111942 (https://doi.org/10.1016/j.jinorgbio.2022.111942)
T. Dimitrijević, I. Novaković, D. Radanović, S. B. Novaković, M. V. Rodić, K. Anđelković, M. Šumar-Ristović, J. Coord. Chem. 73 (2020) 702 (https://doi.org/10.1080/00958972.2020.1740212)
E. R. Sevda, H. Ünver, G. Dikmen, Lett. Org. Chem. 20 (2023) 376 (https://doi.org/10.2174/1570178620666221202090558)
D. Lj. Tomović, A. M. Bukonjić, V. V. Jevtić, Z. R. Ratković, J. V. Bogojeski, A. Đeković, I. D. Radojević, L. R. Čomić, S. B. Novaković, G. A. Bogdanović, S. R. Trifunović, G. P. Radić, S. Cupara, Transit. Met. Chem. 43 (2018) 137 (https://doi.org/10.1007/s11243-018-0201-0)
B. Shaabani, A. A. Khandar, F. Mahmoudi, M. A. Maestro, S. S. Balula, L. Cunha-
-Silva, Polyhedron 57 (2013) 118 (https://doi.org/10.1016/j.poly.2013.04.016)
B. Shaabani, A. A. Khandar, H. Mobaiyen, N. Ramazani, S.S. Balula, L. Cunha-
-Silva, Polyhedron 80 (2014) 166 (https://doi.org/10.1016/j.poly.2014.03.033)
P. Chakrabarti, J. Mol. Biol. 234 (1993) 463 (https://doi.org/10.1006/jmbi.1993.1599)
M. R. Milenković, A. T. Papastavrou, D. Radanović, A. Pevec, Z. Jagličić, M. Zlatar, M. Gruden, G. C. Vougioukalakis, I. Turel, K. Anđelković, B. Čobeljić, Polyhedron 165 (2019) 22 (https://doi.org/10.1016/j.poly.2019.03.001)
R. Bikas, M. S. Krawczyk, T. Lis, ChemistrySelect 5 (2020) 6759 (https://doi.org/10.1002/slct.202001032)
CrysAlis PRO, Oxford Diffraction Ltd., Yarnton, (n.d.)
A. Altomare, G. Cascarano, C. Giacovazzo, A. Guagliardi, J. Appl. Crystallogr. 26 (1993) 343 (https://doi.org/10.1107/S0021889892010331)
G.M. Sheldrick, Acta Crystallogr., C 71 (2015) 3 (https://doi.org/10.1107/S2053229614024218)
A. Rahman, M.I. Choudhary, W.J. Thomson, Bioassay Techniques for Drug Development, Harwood Academic Publishers, Reading, 2001
A. Sartoratto, A. L. M. Machado, C. Delarmelina, G. M. Figueira, M. C. T. Duarte, V. L. G. Rehder, Braz. J. Microbiol. 35 (2004) 275 (https://doi.org/10.1590/S1517-83822004000300001)
B. Meyer, N. Ferrigni, J. Putnam, L. Jacobsen, D. Nichols, J. McLaughlin, Planta Med. 45 (1982) 31 (https://doi.org/10.1055/s-2007-971236)
A. W. Addison, T. N. Rao, J. Reedijk, J. van Rijn, G. C. Verschoor, J. Chem. Soc., Dalton Trans. (1984) 1349 (https://doi.org/10.1039/DT9840001349)
T. Keškić, B. Čobeljić, M. Gruden, K. Anđelković, A. Pevec, I. Turel, D. Radanović, M. Zlatar, Cryst Growth Des. 19 (2019) 4810 (https://doi.org/10.1021/acs.cgd.9b00760)
M. R. Milenković, A. T. Papastavrou, D. Radanović, A. Pevec, Z. Jagličić, M. Zlatar, M. Gruden, G. C. Vougioukalakis, I. Turel, K. Anđelković, B. Čobeljić, Polyhedron 165 (2019) 22 (https://doi.org/10.1016/j.poly.2019.03.001)
C. R. Groom, I. J. Bruno, M. P. Lightfoot, S. C. Ward, Acta Crystallogr., B 72 (2016) 171 (https://doi.org/10.1107/S2052520616003954)
L. J. Farrugia, J. Appl. Crystallogr. 45 (2012) 849 (https://doi.org/10.1107/S0021889812029111)
C. F. Macrae, P. R. Edgington, P. McCabe, E. Pidcock, G. P. Shields, R. Taylor, M. Towler, J. van de Streek, J. Appl. Crystallogr. 39 (2006) 453 (https://doi.org/10.1107/S002188980600731X)
J. E. Anderson, C. M. Goetz, J. L. McLaughlin, M. Suffness, Phytochem. Anal. 2 (1991) 107 (https://doi.org/10.1002/pca.2800020303).