Tuning crystal packing and reactivity through electrostatic and dispersion interactions: The case of phenytoin and its derivatives Scientific paper
Main Article Content
Abstract
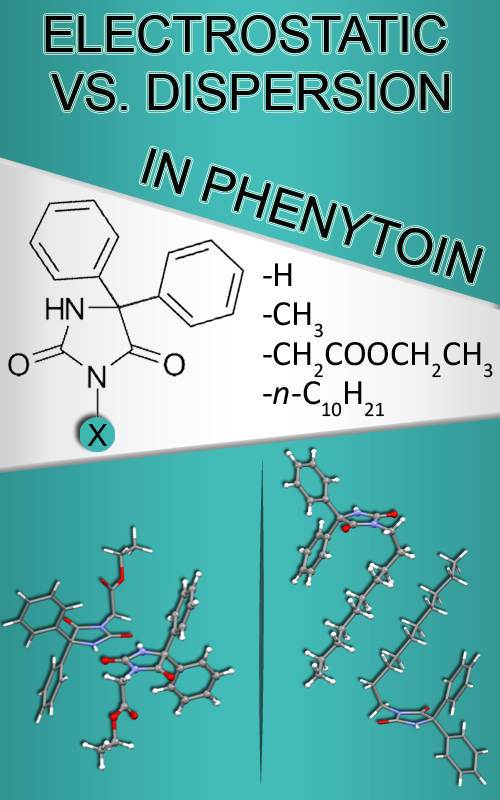
Hydantoin derivatives represent a versatile class of heterocycles, known for their pharmacological properties. Because drug efficacy often depends on the fine-tuning of weak intermolecular (non-covalent) interactions, analysis of the crystal structure of a drug molecule is important, as it enables deciphering its interaction profile. In this study, the crystal packing of phenytoin and its selected derivatives were examined through dimeric motifs with different recognition modes using force-field calculations and a density functional theory (DFT) approach. The relatively polar ethoxyacetyl group at the N3 position of the hydantoin ring, capable of forming hydrogen bonds, enhances the contribution of electrostatic and polar components to the total interaction energy. In contrast, the long alkyl chain promotes hydrophobic contacts, leading to dispersion forces dominating over electrostatic interactions. The reactivity of phenytoin and its derivatives were further evaluated by examining the influence of these substituents using conceptual density functional theory (CDFT) descriptors. These findings demonstrate that substituents significantly affect crystal packing and the balance of non-covalent interactions, providing valuable insights for optimizing molecular recognition and drug–target interactions in the design of new therapeutic agents.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-136/2025-03/200135;451-03-136/2025-03/200026
References
S. Cho, S.-H. Kim, D. Shin, Eur. J. Med. Chem. 164 (2019) 517 (https://doi.org/10.1016/j.ejmech.2018.12.066)
W. Liu, S. Zhang, L. Xiao, Y. Wan, L. He, K. Wang, Z. Qi, X. Li, Pest Manage. Sci. 78 (2022) 1438 (https://doi.org/10.1002/ps.6761)
R. Rayhan, Md. S. H. Shishir, Md. A. Khaleque, Md. R. Amin, Md. R. Ali, M. A. S. Aly, S. M. Ayon, R. Saidur, T. H. Kim, Md. A. Zaed, Md. Z. Hossain, RSC Adv. 15 (2025) 24917 (https://doi.org/10.1039/D5RA03128A)
K. Kaewket, K. Ngamchuea, RSC Adv. 13 (2023) 33210 (https://doi.org/10.1039/D3RA06175B)
E. D. Akpan, O. Dagdag, E. E. Ebenso, Coord. Chem. Rev. 489 (2023) 215207 (https://doi.org/10.1016/j.ccr.2023.215207)
L. L. Brunton, B. C. Knollmann, Goodman & Gilman’s Pharmacological Basis of Therapeutics, 14th ed., L. L. Brunton, B. C. Knollmann, Eds., McGraw-Hill, New York, 2022, p. 1664 (ISBN 978-1264258079)
M. Rask-Andersen, M. Almén, H. Schiöth, Nat. Rev. Drug. Discov. 10 (2011) 579 (https://doi.org/10.1038/nrd3478)
W. Guerrab, El Jemli Meryem, A. Jihane, D. Güneş, T. M. Joel, T. Jamal, I. Azeddine, A. M’Hammed, A. Katim, R. Youssef, J. Biomol. Struct. Dyn. 40 (2021) 8765 (https://doi.org/10.1080/07391102.2021.1922096)
A. Domínguez, A. Álvarez, B. Suárez-Merino, F. Goñi-de-Cerio, Rev. Neurol. 58 (2014) 213 (https://pubmed.ncbi.nlm.nih.gov/24570360/)
H. L.Wong, X. Y. Wu, R. Bendayan, Adv. Drug Deliv. Rev. 64 (2012) 686 (https://doi.org/10.1016/j.addr.2011.10.007)
N. Trišović, N. Valentić, G. Uščumlić, Chem. Cen. J. 5 (2011) 62 (https://doi.org/10.1186/1752-153X-5-62)
J. R. Smythies, in Progress in Drug Research, G. H. Glaser, J. K. Penry, J. Kiffin, D. M. Woodbury, Eds., Raven, New York, 1980, p. 207
N. Trišović, N. Valentić, M. Erović, T. Ðaković-Sekulić, G. Uščumlić, I. Juranić, Monatsh. Chem. 142 (2011) 1227 (https://doi.org/10.1007/s00706-011-0639-7)
N. Trišović, T. Timić, J. Divljaković, J. Rogan, D. Poleti, M. M. Savić, G. Uščumlić, Monatsh. Chem. 143 (2012) 1451 (http://dx.doi.org/10.1007/s00706-012-0791-8)
W. M. Pardridge, Curr. Opin. Pharmacol. 6 (2006) 494 (https://doi.org/10.1016/j.coph.2006.06.001)
M. L. Brown, G. B. Brown, W. J. Brouillette, J. Med. Chem. 40 (1997) 602 (https://doi.org/10.1021/jm960692v)
P. R. Spackman, L. J. Yu, C. J. Morton, M. W. Parker, C. S. Bond, M. A. Spackman, D. Jayatilaka, S. P. Thomas, Angew. Chem. Int. Ed. 58 (2019) 16780 (https://doi.org/10.1002/anie.201906602)
M. Stöhr, T. Van Voorhis, A. Tkatchenko, Chem. Soc. Rev. 48 (2019) 4118 (https://doi.org/10.1039/C9CS00060G)
D. Wu, D. G. Truhlar, J. Chem. Theory Comput. 17 (2021) 3967 (https://doi.org/10.1021/acs.jctc.1c00162)
C. Tantardini, A. A. L. Michalchuk, A. Samtsevich, C. Rota, A. G. Kvashnin, Sci. Rep. 10 (2020) 7816 (https://doi.org/10.1038/s41598-020-64261-4)
S. Tretiakov, A-K. Nigam, R. Pollice, Chem. Rev. 125 (2025) 5776 (https://doi.org/10.1021/acs.chemrev.4c00893)
C. R. Groom, I. J. Bruno, M. P. Lightfoota, S. C. Ward, Acta Crystallogr., B 72 (2016) 171 (https://doi.org/10.1107/S2052520616003954)
Gaussian 09 (revision D.01), Gaussian, Inc., Wallingford, CT, 2009
P. R. Spackman, M. J. Turner, J. J. McKinnon, S. K. Wolff, D. J. Grimwood, D. Jayatilaka, M. A. Spackman, J Appl. Cryst. 54 (2021) 1006 (https://doi.org/10.1107/s1600576721002910)
C. F. Macrae, I. Sovago, S, J. Cottrell, P. T. A. Galek, P. McCabe, E. Pidcock, M. Platings, G. P. Shields, J. S. Stevens, M.Towler, P. A. Wood, J. Appl. Cryst. 53 (2020) 226 (https://doi.org/10.1107/S1600576719014092)
A. Gavezzotti, Acc. Chem. Res. 27 (1994) 309 (https://doi.org/10.1021/ar00046a004)
A. Gavezzotti, G. Filippini, J. Phys. Chem. 98 (1994) 4831 (https://doi.org/10.1021/j100069a010)
K. Chattopadhyay, R. A. Palmer, J. N. Lisgarten, J. Crystallogr. Spectrosc. Res. 23 (1993) 149 (https://doi.org/10.1007/BF01195449)
W. Guerrab, R. Akrad, M. Ansar, J. Taoufik, J. T. Mague, Y. Ramli, IUCrData 2 (2017) x171534 (https://doi.org/10.1107/S2414314617015346)
Y. Ramli, R. Akrad, W. Guerrab, J. Taoufik, M. Ansar, J. T. Mague, IUCrData 2 (2017) x170098 (https://doi.org/10.1107/S2414314617000980)
M. J. Turner, S. P. Thomas, M. W. Shi, D. Jayatilaka, M. A. Spackman, Chem. Comm. 51 (2015) 3735 (https://doi.org/10.1039/C4CC09074H)
M. A. Spackman, D, Jayatilaka, CrystEngComm 11 (2009) 19 (https://doi.org/10.1039/B818330A)
C. F. Mackenzie, P. R. Spackman, D. Jayatilaka, M. A. Spackman, IUCrJ 4 (2017) 575 (https://doi.org/10.1107/S205225251700848X)
P. Geerlings, F. De Proft, W. Langenaeker, Chem. Rev. 103 (2003) 1793 (https://doi.org/10.1021/cr990029p)
P. Politzer, F. Abu-Awwad, Theor. Chem. Acc. 99 (1998) 87 (https://doi.org/10.1007/s002140050307).