N-2 Alkylated analogues of aza-galactofagomine as potential inhibitors of β-glucosidase Scientific paper
Main Article Content
Abstract
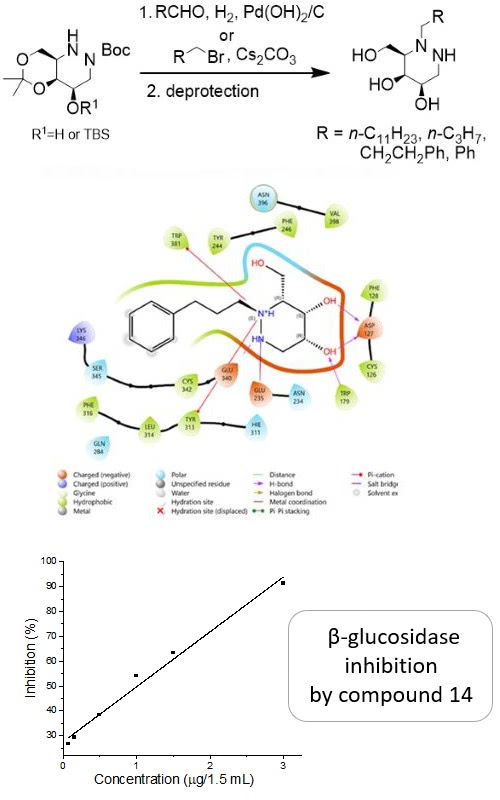
The synthesis of four N-2-alkylated aza-galactofagomine (AGF) analogues was achieved by intermolecular reductive hydrazination or alkylation of suitably protected AGF. The synthesized compounds were evaluated as potential β-glucosidase inhibitors. The preliminary screening of inhibitor activity, conducted with sweet almond β-glucosidase immobilized in agar, as well as the standard inhibition assay with the same enzyme, showed the inhibitory potency of the synthesized analogues. In addition, these results are in a good agreement with the docking analysis of the human acid β-glucosidase, the enzyme implicated in Gaucher’s disease.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.

Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution license 4.0 that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
Funding data
-
Ministarstvo Prosvete, Nauke i Tehnološkog Razvoja
Grant numbers 451-03-65/2024-03/200168;451-03-66/2024-03/200026
References
N. Asano, Glycobiology 13 (2003) 93R (https://doi.org/10.1093/glycob/cwg090)
E. Borges de Melo, A. da Silveira Gomes, I. Carvalho, Tetrahedron 62 (2006) 10277 (https://doi.org/10.1016/j.tet.2006.08.055)
Iminosugars: From Synthesis to Therapeutic Applications, P. Compain, O. R. Martin, Eds., Wiley, Chichester, 2007 (ISBN: 978-0-470-03391-3)
R. J. Nash, A. Kato, C.-Y. Yu, G. W. J. Fleet, Future Med. Chem. 3 (2011) 1513 (https://doi.org/10.4155/fmc.11.117)
N. J. Leidenheimer, Targeting Trafficking in Drug Development. Springer, Cham, 2017, p. 135 (https://doi.org/10.1007/978-3-319-74164-2)
R. E. Boyd, G. Lee, P. Rybczynski, E. R. Benjamin, R. Khanna, B. A. Wustman, K. J. Valenzano, J. Med. Chem. 56 (2013) 2705 (https://doi.org/10.1021/jm301557k)
E. M. Sanchez-Fernandez, J. M. Garcia Fernandez, C. O. Mellet, Chem. Commun. 52 (2016) 5497 (https://doi.org/10.1039/C6CC01564F)
L. Smith, S. Mullin, A. H.V. Schapira, Exp. Neurol. 298 (2017) 180 (https://doi.org/10.1016/j.expneurol.2017.09.010)
J. M. Benito, J. M. García Fernández, C. O. Mellet, Expert Opin. Ther. Pat. 21 (2011) 885 (https://doi.org/10.1517/13543776.2011.569162)
A. Trapero, A. Llebaria, Future Med. Chem. 5 (2013) 573 (https://doi.org/10.4155/fmc.13.14)
H. Martin, L. Ramírez Lázaro, T. Gunnlaugsson, E. M. Scanlan, Chem. Soc. Rev. 51 (2022) 9694 (https://doi.org/10.1039/D2CS00379A)
X. Zhou, Z. Huang, H. Yang, Y. Jiang, W. Wei, Q. Li, Q. Mo, J. Liu, Biomed. Pharmacother. 91 (2017) 504 (https://doi.org/10.1016/j.biopha.2017.04.113)
M. L. Feltri, N. I. Weinstock, J. Favret, N. Dhimal, L. Wrabetz, D. Shin, Glia 69 (2021) 2309 (https://doi.org/10.1002/glia.24008)
A. N. D’Agostino, G. P. Sayre, A. B. Hayles, Arch. Neurol. 8 (1963) 82 (https://doi.org/10.1001/archneur.1963.00460010098012)
C. H. Hill, A. H. Viuff, S. J. Spratley, S. Salamone, S. H. Christensen, R. J. Read, N. W. Moriarty, H. H. Jensen, J. E. Deane, Chem. Sci. 6 (2015) 3075 (https://doi.org/10.1039/C5SC00754B)
A. H. Viuff, H. H. Jensen, Org. Biomol. Chem. 14 (2016) 8545 (https://doi.org/10.1039/C6OB01309K)
J. Marjanovic Trajkovic, Z. Ferjancic, R. N. Saicic, Tetrahedron 73 (2017) 2629 (https://doi.org/10.1016/j.tet.2017.03.052)
S. Pandey, A. Sree, S.S. Dash, D.P. Sethi, BMC Microbiol. 13 (2003) 55 (https://doi.org/10.1186/1471-2180-13-55)
O. G. Korotkova, M. V. Semenova, V. V. Morozova, I. N. Zorov, L. M. Sokolova, T. M. Bubnova, O. N. Okunev, A. P. Sinitsyn, Biochemistry (Moscow) 74 (2009) 569 (https://doi.org/10.1134/S0006297909050137)
R. Lieberman, B. Wustman, P. Huertas, A. C. Powe Jr, C. W. Pine, R. Khanna, M. G. Schlossmacher, D. Ringe, G. A. Petsko, Nat. Chem. Biol. 3 (2007) 101 (https://doi.org/10.1038/nchembio850)
RCSB PDB: Protein Data Bank, https://www.rscb.org
Schrödinger Release 2021-3: Protein Preparation Wizard; Maestro; Epik; Glide, Schrödinger, LLC, New York, 2021
Schrödinger Release 2024-1: Maestro, Schrödinger, LLC, New York, 2024
O. L. Lopez, M. Bols, ChemBioChem 8 (2007) 657 (https://doi.org/10.1002/cbic.200700012)
H. H. Jensen, M. Bols, J. Chem. Soc., Perkin Trans. 1 (2001) 905 (https://doi.org/10.1039/B007973L)
R. Chen, M. Jäättelä, B. Liu, Cancers 12 (2020) 2437 (https://doi.org/10.3390/cancers12092437).